-
胰岛素/葡萄糖代谢信号通路
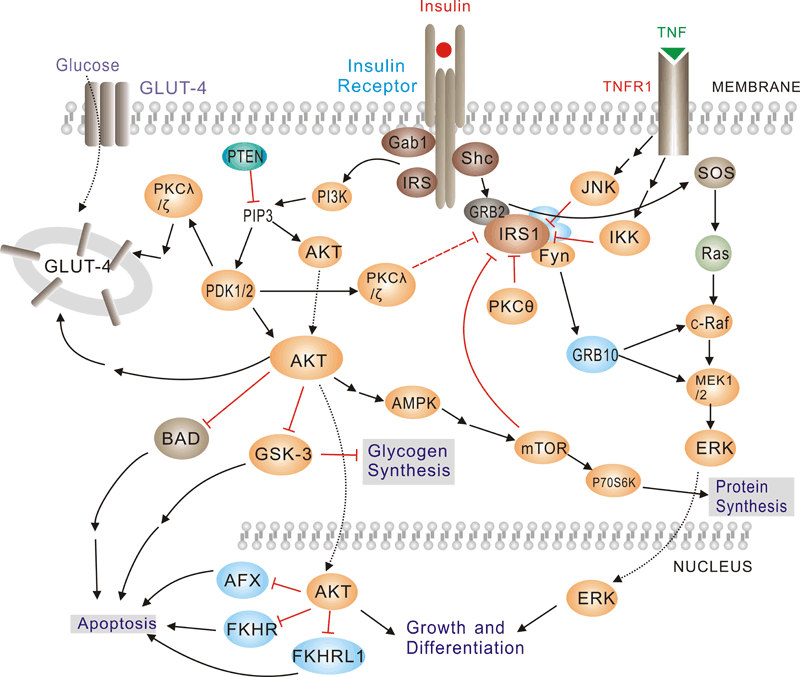 Pathway description:Insulin has diverse effects on cells including stimulation of glucose transport, gene expression and alterations of cell morphology. Insulin signaling begins with either the activation or substrate kinase activity of the insulin receptor (IR), which is the only component of the pathway that is unique to insulin action. The insulin receptor belongs to a subfamily of receptor tyrosine kinases. Activation of the IR can be impaired by post-translational modifications of the protein involving serine phosphorylation, or by binding to inhibiting proteins such as PC-1 or members of the SOCS or Grb protein families. Insulin elicits a diverse array of biological responses by binding to its specific receptor. The hormone mediates these effects by activation of signaling pathways which adaptor molecules such the IRS, the SHC and the GRB2 lipid kinases such as PI3K serine, threonine and tyrosine.
Pathway description:Insulin has diverse effects on cells including stimulation of glucose transport, gene expression and alterations of cell morphology. Insulin signaling begins with either the activation or substrate kinase activity of the insulin receptor (IR), which is the only component of the pathway that is unique to insulin action. The insulin receptor belongs to a subfamily of receptor tyrosine kinases. Activation of the IR can be impaired by post-translational modifications of the protein involving serine phosphorylation, or by binding to inhibiting proteins such as PC-1 or members of the SOCS or Grb protein families. Insulin elicits a diverse array of biological responses by binding to its specific receptor. The hormone mediates these effects by activation of signaling pathways which adaptor molecules such the IRS, the SHC and the GRB2 lipid kinases such as PI3K serine, threonine and tyrosine.
Selected Reviews:ALAN R,SALTIEL,et al.(2001)Insulin signaling and the regulation of glucose and lipid metabolism.Nature.414(6865):799-806.Ogawa W,Matozaki T,et al.(1998)Role of binding proteins to IRS-1 in insulin signaling. Mol Cell Biochem.182(1-2):13-22.Youngren JF. (2007)Regulation of insulin receptor function. Cell Mol Life Sci. 64(7-8):873-91Johnston AM, Pirola L, Van Obberghen E.2003Molecular mechanisms of insulin receptor substrate protein-mediated modulation of insulin signalling. FEBS Lett. 546(1):32-6. -
DNA损伤/修复信号通路
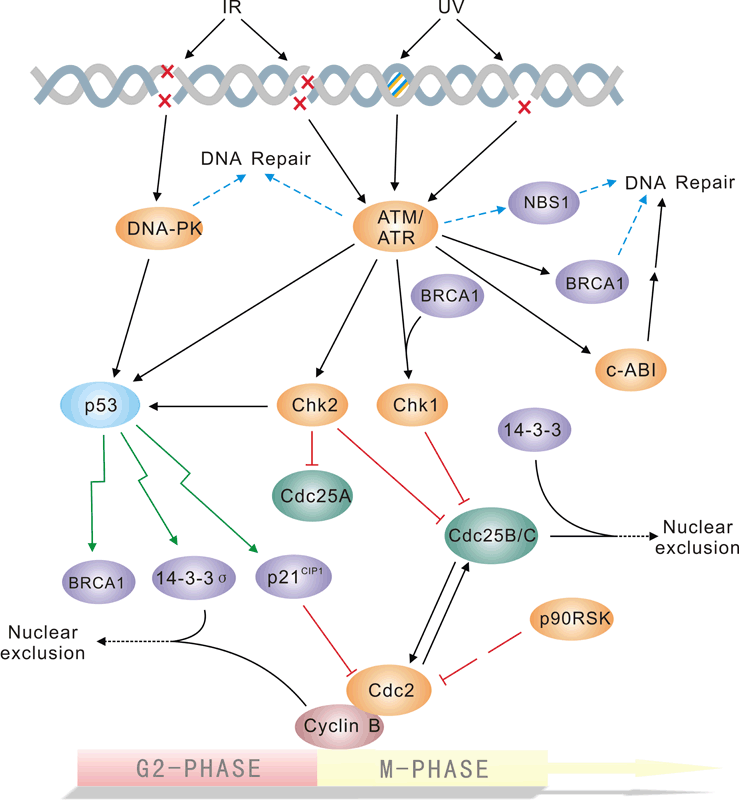
Pathway description:The repair of DNA lesions that occur endogenously or in response to diverse genotoxic stresses is indispensable for genome integrity. DNA lesions activate checkpoint pathways that regulate specific DNA-repair mechanisms in the different phases of the cell cycle. Checkpoint- arrested cells resume cell-cycle progression once damage has been repaired, whereas cells with unrepairable DNA lesions undergo permanent cell-cycle arrest or apoptosis. The G2/M DNA damage checkpoint prevents the cell from entering mitosis (M phase) if the genome is damaged. Environmental DNA-damaging agents include UV light and ionizing radiation. Many of the checkpoint proteins are activated in response to DNA damaged, and a number of phosphorylation events in DNA repair mechanism depend on activation of these kinases. The Mec1/Rad3/ATM/ATR family act early in the checkpoint pathways either as DNA damage detectors or in close association with such detectors. The tumor suppressor P53 is central to the higher eukaryotic checkpoint controls in DNA damage-induced G1 arrest through transcriptional induction of the cyclin-dependent kinases Chk1 and Chk2, and inhibitor p21.Activation of p53 in response to DNA damage and other stresses involves complex posttranslational modification of p53 and its negative modulater MDM2,which is itself induced by p53 at the transcriptional level.Selected Reviews:Norbury CJ,Hickson ID.(2001)Cellular responses to DNA damage.Annu Rev Pharmacol Toxicol.41:367-461.Bartek J,Lukas J.(2003)Chk1 and Chk2 kinases in checkpoint control and cancer.Cancer Cell.3(5)421-9.Branzei D, Foiani M. (2008)Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 9(4):297-308. -
细胞骨架/粘连信号通路
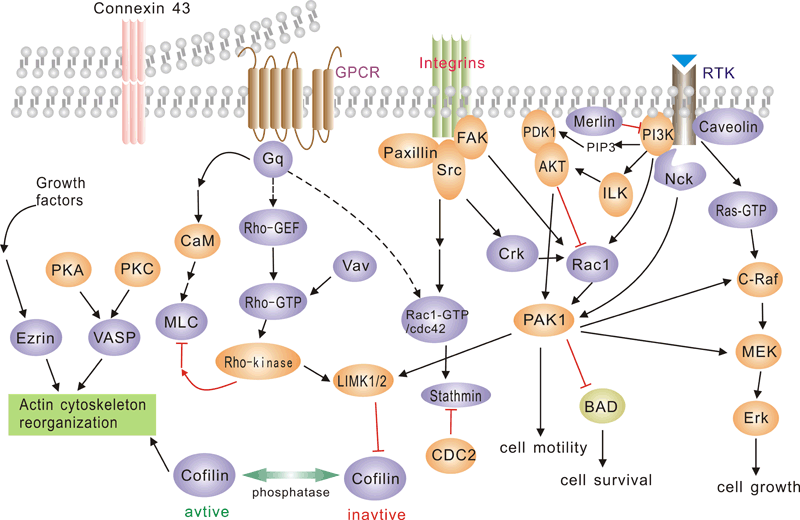 Pathway description:G protein-coupled receptors (GPCRs), comprise a large protein family of transmembrane receptors that sense molecules outside the cell and activate inside signal transduction pathways and, ultimately, cellular responses. GPCRs are activated by an external signal in the form of a ligand or other signal mediator. This creates a conformational change in the receptor, causing activation of a G protein.The extracellular parts of the receptor can be glycosylated. These extracellular loops also contain two highly-conserved cysteine residues that form disulfide bonds to stabilize the receptor structure.The family of p21-activated protein kinases (PAKs) is composed of serine-threonine kinases whose activity is regulated by the small guanosine triphosphatases (GTPases), Rac and Cdc42 In mammalian cells, PAKs have been implicated in the regulation of mitogen-activated protein cascades, cellular morphological and cytoskeletal changes, neurite outgrowth, and cell apoptosis.Focal adhesion kinase (FAK) is a nonreceptor protein tyrosine kinase involved in integrin-mediated control of cell behavior. Following cell adhesion to components of the extracellular matrix, FAK becomes phosphorylated at multiple sites, including tyrosines 397, 576, and 577. Tyr-397 is an autophosphorylation site that promotes interaction with c-Src or Fyn. Tyr-576 and Tyr-577 lie in the putative activation loop of the kinase domain, and FAK catalytic activity may be elevated through phosphorylation of these residues by associated Src family kinase. The clustering of integrins at these sites attracts a large complex of proteins and initiates intracellular regulatory processes, by which such events as cell migration and anchorage-dependent differentiation are controlled. FAK is a protein tyrosine kinase which is recruited at an early stage to focal adhesions and which mediates many of the downstream responses. FAK subsequently interacts with a number of down-stream signalling proteins, including the adaptor protein Grb2 and the p85 _-subunit of phosphatidylinositol 3 kinase (PI3 kinase)References:Daniels, R. H. & Bokoch, G. M. (1999) p21-Activated protein kinase: a crucial component of morphological signaling? Trends Biochem. Sci. 24, 350-355.Lei, M. et al. (2000) Structure of PAK1 in an Autoinhibited Conformation Reveals a Multistage Activation Switch . Cell 102, 387-397Joost P, Methner A(2002) Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol 3 (11), research0063.1-0063.16.Bjarnadottir TK. et al. (2006) Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 88 (3), 263-73.Astier A. et al. (1997) The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J Biol Chem.272,228–232.Avraham S,.et al. (1995) Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 270,27742–27751.
Pathway description:G protein-coupled receptors (GPCRs), comprise a large protein family of transmembrane receptors that sense molecules outside the cell and activate inside signal transduction pathways and, ultimately, cellular responses. GPCRs are activated by an external signal in the form of a ligand or other signal mediator. This creates a conformational change in the receptor, causing activation of a G protein.The extracellular parts of the receptor can be glycosylated. These extracellular loops also contain two highly-conserved cysteine residues that form disulfide bonds to stabilize the receptor structure.The family of p21-activated protein kinases (PAKs) is composed of serine-threonine kinases whose activity is regulated by the small guanosine triphosphatases (GTPases), Rac and Cdc42 In mammalian cells, PAKs have been implicated in the regulation of mitogen-activated protein cascades, cellular morphological and cytoskeletal changes, neurite outgrowth, and cell apoptosis.Focal adhesion kinase (FAK) is a nonreceptor protein tyrosine kinase involved in integrin-mediated control of cell behavior. Following cell adhesion to components of the extracellular matrix, FAK becomes phosphorylated at multiple sites, including tyrosines 397, 576, and 577. Tyr-397 is an autophosphorylation site that promotes interaction with c-Src or Fyn. Tyr-576 and Tyr-577 lie in the putative activation loop of the kinase domain, and FAK catalytic activity may be elevated through phosphorylation of these residues by associated Src family kinase. The clustering of integrins at these sites attracts a large complex of proteins and initiates intracellular regulatory processes, by which such events as cell migration and anchorage-dependent differentiation are controlled. FAK is a protein tyrosine kinase which is recruited at an early stage to focal adhesions and which mediates many of the downstream responses. FAK subsequently interacts with a number of down-stream signalling proteins, including the adaptor protein Grb2 and the p85 _-subunit of phosphatidylinositol 3 kinase (PI3 kinase)References:Daniels, R. H. & Bokoch, G. M. (1999) p21-Activated protein kinase: a crucial component of morphological signaling? Trends Biochem. Sci. 24, 350-355.Lei, M. et al. (2000) Structure of PAK1 in an Autoinhibited Conformation Reveals a Multistage Activation Switch . Cell 102, 387-397Joost P, Methner A(2002) Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol 3 (11), research0063.1-0063.16.Bjarnadottir TK. et al. (2006) Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 88 (3), 263-73.Astier A. et al. (1997) The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J Biol Chem.272,228–232.Avraham S,.et al. (1995) Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 270,27742–27751. -
染色质/转录信号通路
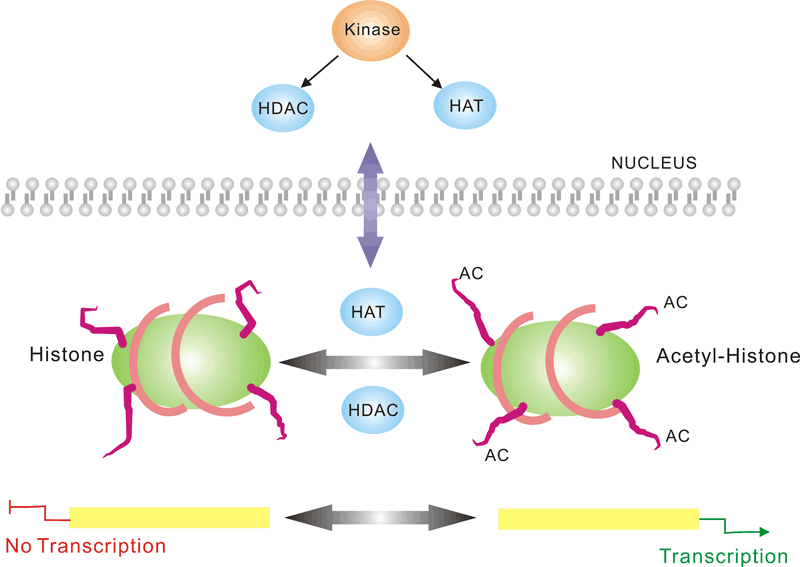
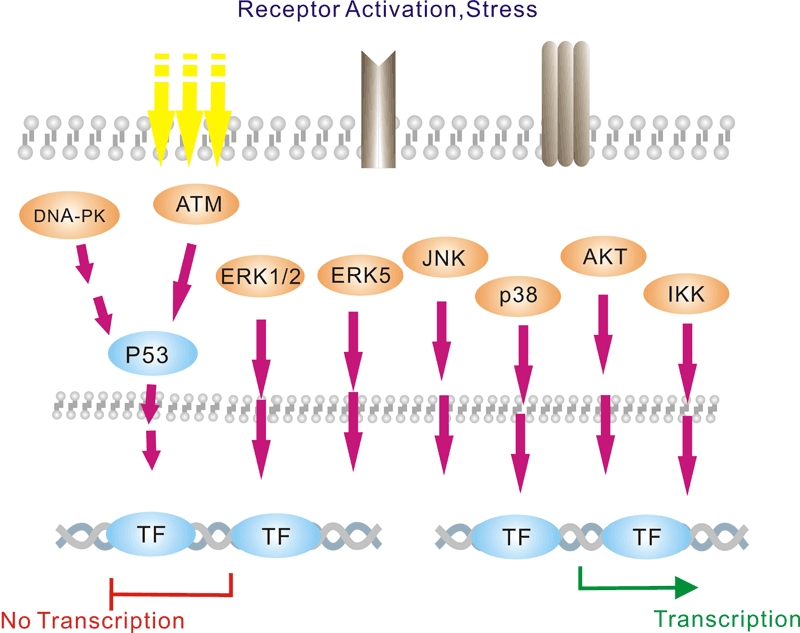 Pathway description:Chromatin is the substance which becomes visible chromosomes during cell division. Its basic unit is nucleosome, composed of 146 bp DNA and eight histone proteins. The structure of chromatin is dynamically changing, at least in part, depending on the need of transcription. In the metaphase of cell division, the chromatin is condensed into the visible chromosome.Transcription is the process through which a DNA sequence is enzymatically copied by an RNA polymerase to produce a complementary RNA. In eukaryotes, it takes place in the nucleus, mitochondria and chloroplast.Protein acetylation plays a crucial role in regulating chromatin structure and transcriptional activity. Acetylation complexes or deacetylation complexes can be recruited to DNA-bound transcription factors (TFs) in response to signaling pathways. Histone hyperacetylation by histone acetyltransferases (HATs) is associated with transcriptional activation, presumably by remodeling nucleosomal structure into an open conformation more accessible to transcription complexes. Conversely, histone deacetylation by deacetylation complexes (such as HDAC) is associated with transcriptional repression reversing the chromatin remodeling process.Several transcriptional coactivators and corepressors possess intrinsic acetylase or deacetylase enzymatic activities, respectively. Site-specific acetylation of a growing list of non-histone proteins, including p53 and E2F, has been shown to play an important role in transcriptional regulation and cell proliferation.
Pathway description:Chromatin is the substance which becomes visible chromosomes during cell division. Its basic unit is nucleosome, composed of 146 bp DNA and eight histone proteins. The structure of chromatin is dynamically changing, at least in part, depending on the need of transcription. In the metaphase of cell division, the chromatin is condensed into the visible chromosome.Transcription is the process through which a DNA sequence is enzymatically copied by an RNA polymerase to produce a complementary RNA. In eukaryotes, it takes place in the nucleus, mitochondria and chloroplast.Protein acetylation plays a crucial role in regulating chromatin structure and transcriptional activity. Acetylation complexes or deacetylation complexes can be recruited to DNA-bound transcription factors (TFs) in response to signaling pathways. Histone hyperacetylation by histone acetyltransferases (HATs) is associated with transcriptional activation, presumably by remodeling nucleosomal structure into an open conformation more accessible to transcription complexes. Conversely, histone deacetylation by deacetylation complexes (such as HDAC) is associated with transcriptional repression reversing the chromatin remodeling process.Several transcriptional coactivators and corepressors possess intrinsic acetylase or deacetylase enzymatic activities, respectively. Site-specific acetylation of a growing list of non-histone proteins, including p53 and E2F, has been shown to play an important role in transcriptional regulation and cell proliferation.
Selected Reviews:
Davie JR, Samuel SK, Spencer VA, et al. (1999)Organization of chromatin in cancer cells: role of signalling pathways. Biochem Cell Biol.77(4):265-75.Spencer VA, Davie JR.(2000)Signal transduction pathways and chromatin structure in cancer cells. J Cell Biochem Suppl. 35:27-35.Crosio C, Heitz E, Allis CD,et al. (2003)Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J Cell Sci. 116(Pt 24):4905-14. -
细胞周期信号通路
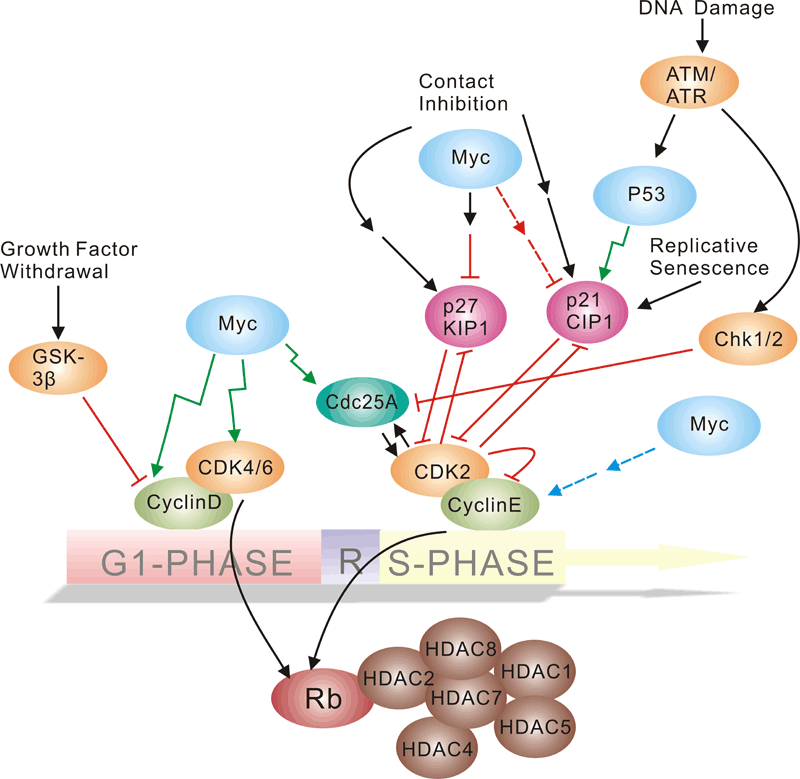
Pathway description:Cell-division control affects many aspects of development. Caenorhabditis elegans cell-cycle genes have been identified over the past decade, including at least two distinct Cyclin-Dependent Kinases (CDKs), their cyclin partners, positive and negative regulators, and downstream targets. The balance between CDK activation and inactivation determines whether cells proceed through G1 into S phase, and from G2 to M, through regulatory mechanisms that are conserved in more complex eukaryotes. Many different stimuli exert checkpoint control including TGF, DNA damage, contact inhibition, replicative senescence, and growth factor withdrawal. G1 phase CDKs and their inhibitors (CKIs) are central to the pathways that regulate commitment to cellular division in response to positive as well as negative growth effectors. Many checkpoints are deregulated in oncogenesis, and this is often due to alterations in cyclin-CDK complexes.CDK activity is modulated by cyclin binding, phosphorylation, and CKIs, including the INK4 proteins and the closely related inhibitors p21Cip1 and p27Kip1. The downstream targets of CDKs and their modulation by TGF-beta and other growth factors include proteins of the retinoblastoma family, and the related modulation of the transcriptional activity of the E2F family members.Selected Reviews:Dyson, N. (1998) The regulation of E2F by pRB-family proteins. Genes Dev. 12, 2245–2262.Ravitz MJ, Wenner CE. (1997)Cyclin-dependent kinase regulation during G1 phase and cell cycle regulation by TGF-beta. Adv Cancer Res. 71:165-207.van den Heuvel S.(2005) Cell-cycle regulation. WormBook. 21:1-16. -
癌症/细胞凋亡信号通路
Pathway description:
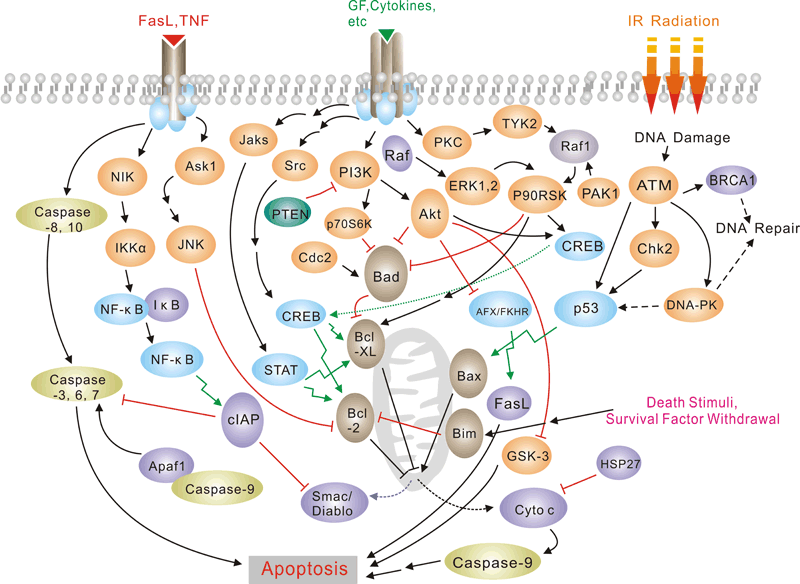
Apoptosis is a naturally occurring process by which a cell is directed to Programmed Cell Death. Apoptosis is based on a genetic program that is an indispensable part of the development and function of an organism and is a regulated physiological process leading to cell death. In apoptosis pathways, signaling results in the activation of a family of Cysteine Proteases, named Caspases that act in a proteolytic cascade to dismantle and remove the dying cell,are central regulators of apoptosis.Initiator caspases (including 8, 9,10) are closely coupled to pro-apototic signals. Once activated, these caspases cleave and activate downstream effector caspases (3,6,7) which in turn cleave cytoskeletal and nuclear proteins and induce apoptosis. Cytochrome C released from damaged mitochondria is coupled to the activation of caspase 9.Pro-apoptotic stimuli include the FasL, TNF, DNA damage. Fas and TNFR activate caspases 8 and 10; DNA damage leads to the activation of caspase 9. Anti-apoptotic ligands including growth factors and cytokines activate AKT and p90RSK, which inhibit Bad and prevent cytochrome C release. TNFR can also stimulate an anti-apoptotic pathway by inducing IAP, which directly inhibits caspases 3, 7 and 9.Alternatively, apoptosis is inhibited via an adaptor protein complex which activates NF-kB and induces survival genes including IAP. The Bcl-2 family of proteins regulate apoptosis by controlling mitochondrial permeability and the release of cytochrome C. The anti-apoptotic proteins Bcl-2 and Bcl-xL reside in the outer mitochondrial wall and inhibit cytochrome C release. The pro- apoptotic Bcl-2 proteins Bad, Bid, Bax and Bim reside in the cytosol but translocate to mitochondria following death signaling, where they promote the release of cytochrome C. Bad translocates to mitochondria and forms a pro-apoptotic complex with Bcl-xL. This translocation is inhibited by survival factors that induce the phosphorylation of Bad, leading to its cytosolic sequestration. Bax and Bim translocate to mitochondria in response to death stimuli, including survival factor withdrawal. Bcl-xL, Bcl-2 and Bax apparently influence the voltage-dependent anion channel (VDAC), which can control cytochrome C release. p53, activated following DNA damage, induces the transcription of Bax. Released cytochrome C binds Apaf1 and forms an activation complex with caspase9.Selected Reviews:Czerski L,Nune G..(2004)Apoptosome formation and Csapase activation:is it different in the heart.J Mol Cardiol.37(13),643-652.Debatin KM, Krammer PH (2004) Death receptors in chemotherapy and cancer. Oncogene 23(16), 2950–66. -
流式细胞术(FC)
Solutions and Reagents1. PBS: Phosphate Buffered Saline2. Incubation Buffer: PBS with 0.5%BSA3. Wash buffer: PBS with 1% BSA(or 1% FBS)4. Cell fixation: 2% formaldehyde solution5. RBC lysis buffer: Red Blood Cell Lysis BufferFlow Cytometry(FC) Protocol For Peripheral Blood Sample1. Aliquot 1 x 106 cells by volume in a assay tube per test from peripheral blood sample which was
anticoagulated by EDTA.2. Add 2–3ml wash buffer to each tube and rinse by centrifugation. Repeat.3. Resuspend cells in 100 µl incubation buffer in a 5ml assay tube per test.4. Add fluorochrome, or biotinylated-conjugated primary antibody at the appropriate dilution (see the
antibody datasheet for the appropriate dilution, such as 10 μl/Test) to the assay tubes.5. After mixing, incubate for 20 minutes at room temperature away from light.6. Add 2ml 1×RBC lysis buffer to the tube, after mixing incubate it for 10 minutes away from light for
dissolution of red blood cells.7. Discard the supernatant by centrifugation and repeat wash by centrifugation in 2–3 ml wash buffer buffer.8. Resuspend cells in 0.5 ml PBS and analyze on flow cytometer.Flow Cytometrys(FC) Protocol For Cells Sample1. Aliquot 1 x 106 cells from pretreated sample in 100ul by volume in a 5ml assay tube per test.2. Add fluorochrome, or biotinylated-conjugated primary antibody at the appropriate dilution (see the
antibody datasheet for the appropriate dilution, such as 10 μl/Test) to the assay tubes.3. Wash by centrifugation in 2–3 ml wash buffer.4. Resuspend cells in 0.3-0.5 ml PBS and analyze on flow cytometer.Notices:Since applications vary, results can be optimized through titrating the appropriate dilution by analyzer. -
免疫荧光法(IF)
Immunofluorescence Solutions and Reagents
A. MOWIOL (anti-fade agent) Calbiochem # 475904
1. Place 6 grams glycerol in a 50 mL tube containing a small stirring bar.
2. Add 2.4 grams Mowiol and stir to mix.
3. While stirring add 6 mL ultrapure water and leave 2 hours at room temp.
4. Add 12 mL 0.2 M Tris, pH 8.5 and 230 mL 1 %Thimerosal (w/v in water).
5. Incubate in hot water (50 °C) for 10 min. with frequent stirring.
6. Centrifuge at 5000 g for 15 min. to clarify stirring to dissolve the Mowiol. This can be repeated over
several hours to get most of the Mowiol into solution. Store as 2 mL aliquots in glass tubes at -20 °C.
These are stable for about 1 year.
Warm tube to room temperature before use. After use, store at 4 °C for about 1 month. Discard once crystalline deposits are seen in slides or tubes.
Immunofluorescence Protocol
1. Plate adequate number of cells into 8-well Lab-Tek. II - Chamber Slide. System least 24 hours before
fixation.
2. Aspirate medium from wells.
3. Wash with 1XPBS pH7.4 for two times.
4. Fix the cells by adding 250ul pre-cold methanol in each well in -20 °C and incubate for 20 min at -20 °C.
5. Wash the wells once with 0.5 mL PBS, then aspirate. Add a second 0.5 mL PBS to each well and let
incubate 5 min.
6. Add 200ul 1% BSA in PBS to each well and incubate 1.5 hours at room temperature.
7. Wash the cells twice with PBS and aspirate medium.
8. Add 150ul primary Ab (in PBS + 1% BSA) to each well and incubate for 2 hours at 37°C or 4 °C
overnight.
9. Wash the cells with 0.5 mL PBS with gentle shaking for 5 min. at room temperature (do this 3 times).
The Following steps should be performed with as little exposure to light as possible
10. ncubate the cells with 200ul appropriate secondary Ab conjugated to fluorophore Alexa594 or Alexa
488 from Invitrogen (in PBS + 1% BSA) and incubate for 20 min at room temperature.
11. Dyes such as Hoechst (diluted from stock to stain the nucleus (10 mg/mL Hoechst stock solution) of
this incubation to make the final concentration 1.5ug/ml.
12. Wash the cells 3 times with 0.5 mL PBS with gentle shaking for 5 min.
13. Aspirate as much PBS out of the wells as possible.
14. Place a drop of mounting medium (Mowiol solution brought to room temperature) on to the middle of the
Chamber Slide. System.
15. Put appropriate glass coverslips on the top the plate.
16. Let slides dry at room temperature (overnight in dark) and examine the cells under a fluorescence
microscope.
-
免疫组化(IHC)
操作步骤
(推荐#CK0062 免疫组化试剂盒),CK0063免疫组化笔
1、组织固定:在脱蜡之前,将切片 60°C 恒温箱中烘烤 60 分钟。
2、脱蜡:将切片用二甲苯浸泡 10 分钟,更换二甲苯后再浸泡 10 分钟。
3、水化:分别用无水乙醇浸泡 5 分钟;95%乙醇中浸泡 5 分钟;85%乙醇中浸泡5 分钟;75%乙醇中浸泡5分钟。
4、洗涤:ddH2O 浸泡 5 分钟,清洗 3 次。
5、抗原修复(煮沸法):压力锅中加入足以淹没切片的 10 mmol/L 的枸橼酸盐缓冲液(pH 6.0)(配制:将复性剂溶于相应体积 ddH2O 中,混匀),加热至沸腾,切片置耐热塑料切片架上,放入锅中,盖好锅盖,扣上压力阀,继续加热,设置保压 3 分钟,时间到后打开放气阀放气,压力归零后开锅盖将内锅取出放置室温冷却,待溶液冷却至室温后取出切片(约 30 分钟)。
6、洗涤:ddH2O 浸泡 5 分钟,清洗 2 次,PBST 浸泡 5 分钟,清洗 2 次。 www.sabbiotech.com 成份 20 片 50 片复性剂(溶解前请混匀) 6 g (溶于 2 L ddH2O) 15 g(溶于 5 L ddH2O)HRP 标记的二抗(抗兔/小鼠) 500 μl 1.25 ml DAB 缓冲液(20X) 50 μl 125 μl DAB 底物(20X) 50 μl 125 μl DAB 色原(20X) 50 μl 125 μl 苏木素体细胞快速染色液 1 ml 2.5 ml For Research Use Only Order:order@signalwayantibody.com Support:tech@signalwayantibody.com
7、灭活酶:每张切片滴加 50 μl 灭活酶试剂,室温避光处理 15 分钟。
8、洗涤:PBST 浸泡 5 分钟,清洗 3 次。(第一次迅速倒掉)
9、封闭:每张切片滴加 3% BSA-PBST 50 μl,湿盒中室温封闭 30 分钟。
10、加一抗:弃去封闭液,每张切片滴加 50 μl 一抗,4°C 湿盒中孵育过夜或 37°C 1 小时。
11、复温:次日取出切片,室温复温 40 分钟。(复温仅针对一抗 4°C 孵育过夜,37℃孵育直接进行下一步)
12、洗涤:PBST 浸泡 5 分钟,清洗 3 次。(第一次迅速倒掉)
13、加酶标二抗:每张切片滴加 HRP 标记的二抗(抗兔/小鼠)25 μl,室温或37°C 孵育30 分钟。
14、洗涤:PBST 浸泡 5 分钟,清洗 3 次。(第一次迅速倒掉)
15、显色(DAB 法):每张切片滴加 50 μl 显色液(显色液配制:85% ddH2O+ 5% DAB 缓冲液+5%DAB底物+5%DAB色原,避 光现配现用,按需要量配制),湿盒中显色 5 分钟。
16、终止显色:用蒸馏水终止显色反应。
17、复染:每张切片滴加 50 μl 苏木素体细胞快速染色液,染色 5 分钟,用蒸馏水冲洗干净。
18、脱色反蓝:将切片放入 1%盐酸~乙醇中脱色 3~5 秒后迅速取出放入蒸馏水中终止,再放入PBST 中反蓝5分钟。
19、封片:分别在 75%乙醇中浸泡 5 分钟;85%乙醇中浸泡 5 分钟;95%乙醇中浸泡5 分钟;无水乙醇中浸泡5分钟。用二甲苯浸泡 10 分钟,更换二甲苯后再浸泡 10 分钟。之后在切片上加中性树胶,加盖玻片。
20、观察:显微镜。
注意事项
抗原修复后需冷却,避免洗涤时脱片。
加抗体时避免交叉污染,导致假阳性。
显色时间可根据实际染色效果来确定,不一定遵循规定的时间。
封片时不要产生气泡,以免影响观察。
显微镜观察时可在低倍镜下先找好视野,再换高倍镜拍照。
如应用于冰冻切片,请用 4°C 丙酮固定 10 分钟,然后从第 6 步开始操作。
De-Paraffinization and Antigen Retrieval
1. Incubate slide at 60°C for 60 min.
2. Bath the slides in antigen retrieval solution and heat it to boil with a microwave on medium power.
Once boiling, immediately transfer to a small power and keep faint boiling while heating for 2-3 min.
3. Cool them to room temperature and wash rocking in distilled water several times.
4. Immerse slides in 3% H2O2 (in fresh methanol) for 15 min at room temperature.
5. Wash with TBS (pH 7.6) three times, 5 min each time.
Staining with Primary Antibody
1. Dilute primary antibody with 3% BSA in TBS. Cover the tissue section on the slide with diluted primary
antibody (use 50 – 150μl for each slide).
2. Incubate at 37°Cfor 2 hours (The optimal incubation time, incubation temperature, and antibody dilution
should be determined by the individual laboratory).
3. Wash with TBS three times, 5 min each time.
Staining with Secondary Antibody
1. Incubate with 100-200μl Polymer Enhancer. Incubate 30 min at room temperature.
2. Wash with TBS for 3 times, 5 min each time.
3. Incubate with 100-200μl Polymerized HRP and incubate 30 min at 37°C.
4. Wash with TBS for 3 times, 5 min each time.
5. Add DAB solution and incubate 3-5 min(The reaction progress and the optimal time should be determine
according to microscope).
6. Wash with distilled water for 2 times, 5 min each time.
7. Counterstain sections in hematoxylin if required,wash with distilled water.Immerse slides in 0.1% HCl-
ethanol for 1-10 seconds, wash with distilled water. Doing a counterstain of sections in hematoxylin for
1-2 min and wash them with distilled water.
8. Dehydrate through 100% ethanol for 5 min, 90% ethanol for 5 min, 75% ethanol for 5 min, and coverslip
with mounting medium.
-
蛋白免疫印迹杂交(WB)
背景介绍
蛋白免疫印迹( Western Blot)是将PAGE(聚丙烯酰胺凝胶电泳)分离的蛋白质样品,转移到固相载体(例如硝酸纤维素薄膜)上,固相载体以非共价键形式吸附蛋白质,且能保持电泳分离的多肽类型及其生物学活性不变。以固相载体上的蛋白质或多肽作为抗原,与对应的抗体起免疫反应,再与酶或同位素标记的第二抗体起反应,经过底物显色或放射自显影以检测电泳分离的特异性目的基因表达的蛋白成分。
一. 仪器与材料
1. 仪器
1) 电泳仪 2)电转仪 3)摇床 4)化学发光成像仪
2. 材料
1) 30%丙烯酰胺溶液
2) 1.5 mol/L Tris (pH8.8)
3) 1.0 mol/L Tris (pH6.8)
4) 10%SDS
5) 10%过硫酸胺
6) TEMED
7) 5× 上样缓冲液
8) 5× SDS电泳缓冲液
9) 10×转移缓冲液
10) NC膜
11) 洗涤液:TBST(1×TBS,0.1%TWEEN20)
12) 封闭液,抗体稀释液:5%脱脂奶粉的TBST
13) HRP-羊抗兔IgG
14) ECL底物液A
15) ECL底物液B
二. 操作步骤
1. 组装制胶平台
玻璃板用自来水清洗干净,晾干。去离子水检漏后,用滤纸吸去玻璃板中残留的水。
2. 配制所需浓度分离胶(配方见附表)
注意:如果发现10%SDS有沉淀,可先放置50℃左右的温箱或温水浴中至沉淀溶解,待其冷却后方能配胶; 胶液配好后要充分混匀。
3. 灌分离胶
灌胶时要防止气泡产生,每次不要把枪头中液体完全打尽,以免带进气泡,一块0.75mm厚度的胶需加入约4ml的分离胶,灌完后在胶面上轻轻加入2ml蒸馏水封胶。室温凝胶30-40分钟。胶凝后将封胶水倒掉,并用滤纸吸干微量剩余的水。
4. 配制 5% 浓缩胶(配方见附表)
5. 灌浓缩胶
将浓缩胶加满至整个制胶平板,插上合适的梳子。室温凝胶30分钟后。将玻璃板取出,并将玻璃板反转置于内侧。在电泳缓冲液中将梳子拔出。
6. 样品准备
同时进行样品处理 (样品与5×上样缓冲液按照体积比2: 1混合),100℃煮沸5分钟。将处理好的样品放置4℃冰箱预冷5分钟。如有沉淀,则将样品离心弃沉淀。
7. 上样
在电泳芯内层玻板之间灌满电泳缓冲液;依次点上样品及Marker。
8. 电泳:90V恒压跑浓缩胶,然后120 V恒压跑分离胶
9. 转膜准备
剪好大小与胶相同的1张NC膜和4层滤纸,将膜取出(要用镊子,不可以用手直接接触膜表面)先在水中浸泡10分钟,以除去气泡;然后置于1×转移缓冲液中平衡20分钟。再将滤纸于1×转移缓冲液中浸透平衡,时间不宜过长。按照滤纸-凝胶-膜-滤纸三明治式放好。胶和膜之间不能有气泡。膜在正极,胶在负极。
10. 转膜
检测蛋白分子量
转膜条件
>200kd
200mA过夜,(70V)
100-200kd
150 mA过夜,(60V)或200mA,1.5h~2h
30-100kd
200mA,1h-1.5h,(70V)或100mA过夜(35V)
<30kd
200mA,45min-1h(70V)
将预冷的转移缓冲液加入到转印槽中,按所需条件调好仪器参数,在放置了冰块的水中进行转膜。
11. 收膜和封闭
转膜终止,取出NC膜并用TBST清洗,做好标记后可根据需要置于立春红染液中观察转膜效果,观察完用TBST清洗,然后放入加有封闭液的容器中,室温封闭1 hr左右。封闭后用TBST清洗,或晾干备用保存至-20℃冰箱。
12. 切膜
将转好的膜用TBST先浸泡透,取出后将转有蛋白的一面朝上放置在平板上,用刀按方案所需将膜裁切,并在无蛋白位置作好标记(包括抗体编号,膜编号等),分别放入到各反应槽中。
(磷酸酶实验CIP)
用水稀释10x 磷酸酶buffer至1x工作液,以每个泳道50单位CIP配5ml工作液的体系孵膜,37℃孵
育2小时。将孵完CIP的膜在TBST中洗净。
13. 孵育一抗
将各个反应槽中加入所需反应体积的5%脱脂奶粉TBST,然后按照设计方案的稀释度分别加入相应体积的一抗样品。4℃缓慢摇晃过夜。
14. 洗涤
在各个反应槽中加入适量的TBST,摇床摇动5分钟,然后将TBST倒掉,重复3次。
15. 孵育二抗
配制二抗孵育液:将HRP-羊抗兔IgG二抗按照工作稀释比例加到5%脱脂奶粉TBST中,摇床混匀5分钟。将洗涤过的NC膜全部取出,转移到二抗孵育液中室温1小时。
16. 洗涤
倒掉二抗孵育液,加入适量TBST,摇床摇动5分钟,然后将TBST倒掉,重复3次。
17. 底物反应
配制ECL反应底物:临用前,将ECL底物液A 和ECL底物液B等体积混合并混匀,避光保存备用。
将配好的反应底物液均匀的滴加在膜上,保证所有膜都被反应液完全覆盖后,反应1-2分钟,注意膜的位置不要有气泡。
18. 曝光
预先打开凝胶成像仪,调节至最佳条件,将放有膜的平板放入暗箱内,开始曝光。根据情况选择最适的曝光时间。
19. 结果保存
选择最佳图片,整理归档。
-
Akt signaling pathway
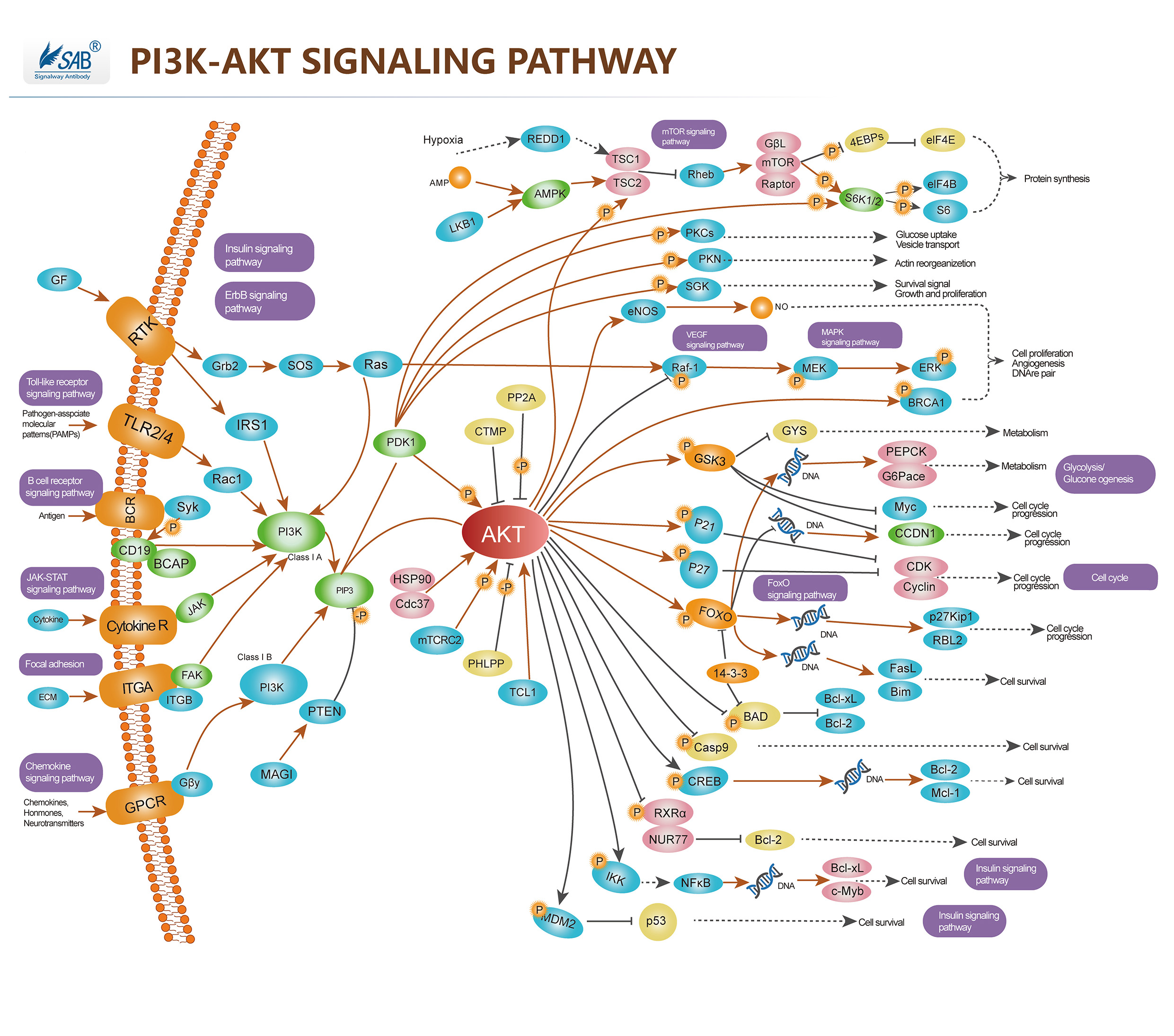 Pathway description:Akt (v-Akt Murine Thymoma Viral Oncogene)/ PKB (Protein Kinase-B) is a Serine/threonine Kinase that is involved in mediating various biological responses, by phospho-rylation of a number of intracellular proteins, regulates different cellular processes, such as cell growth, cell cycle, apoptosis and glucose metabolism. Akt is activated by several hormones including insulin, growth factors, by signals derived from receptors for extracellular matrix molecules such as integrins, by several forms of cellular stress such as oxidative stress or cell swelling, and by activation of Ras. Three mammalian isoforms are currently known: Akt1/PKB- Alpha, Akt2/PKB-Beta and Akt3/ PKB-Gamma. All three isoforms of Akt share a common structure of three domains. Activation of PI3K by receptor tyrosine kinases mediates the phosphorylation of PIP2 to PIP3 and thereby the recruitment of Akt/PKB and PDK to the plasma membrane. Akt/PKB is subsequently activated and phosphorylation at Thr308 by PDK. PIP3 at the cell membrane recruits protein kinases such as Akt/PKB and PDK.PTEN functions as an antagonist of PI3K.PDK further activates SGK and aPKC , aPKC and SGK in turn phosphorylate a wide variety of cellular signaling molecules relevant for the regulation of cell growth, cell cycle and cell proliferation, including FKHR, GSK3, mTOR and p70S6K, for apoptosis, including Bad, caspase 9, IκB, FKHR, Mdm2. Akt/PKB further activates mTOR, a kinase stimulating the uptake of nutrients such as glucose, amino acids, cholesterol and iron. mTOR regulates the phosphorylation of p70S6K which can similarly be activated by PDK. mTOR further activates eIF4E-binding protein-1 and thus is involved in the regulation of translation.
Pathway description:Akt (v-Akt Murine Thymoma Viral Oncogene)/ PKB (Protein Kinase-B) is a Serine/threonine Kinase that is involved in mediating various biological responses, by phospho-rylation of a number of intracellular proteins, regulates different cellular processes, such as cell growth, cell cycle, apoptosis and glucose metabolism. Akt is activated by several hormones including insulin, growth factors, by signals derived from receptors for extracellular matrix molecules such as integrins, by several forms of cellular stress such as oxidative stress or cell swelling, and by activation of Ras. Three mammalian isoforms are currently known: Akt1/PKB- Alpha, Akt2/PKB-Beta and Akt3/ PKB-Gamma. All three isoforms of Akt share a common structure of three domains. Activation of PI3K by receptor tyrosine kinases mediates the phosphorylation of PIP2 to PIP3 and thereby the recruitment of Akt/PKB and PDK to the plasma membrane. Akt/PKB is subsequently activated and phosphorylation at Thr308 by PDK. PIP3 at the cell membrane recruits protein kinases such as Akt/PKB and PDK.PTEN functions as an antagonist of PI3K.PDK further activates SGK and aPKC , aPKC and SGK in turn phosphorylate a wide variety of cellular signaling molecules relevant for the regulation of cell growth, cell cycle and cell proliferation, including FKHR, GSK3, mTOR and p70S6K, for apoptosis, including Bad, caspase 9, IκB, FKHR, Mdm2. Akt/PKB further activates mTOR, a kinase stimulating the uptake of nutrients such as glucose, amino acids, cholesterol and iron. mTOR regulates the phosphorylation of p70S6K which can similarly be activated by PDK. mTOR further activates eIF4E-binding protein-1 and thus is involved in the regulation of translation.
Selected Reviews:Wang Q,Liu L,et al.(2003)Control of synaptic strength,a novel function of Akt.Neuron.38(6),915.Basso Ade, Solit DB,et al.(2002)Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function.J Biol Chem.277(2),39858.Fornaro M,Plescia J,et al.(2003)Fibronectin protects prostate cancer cells from tumor necrosis factor-alpha-induced apoptosis via the AKT/survivin pathway. J Biol Chem.278(50),50402.Pommery N,Henichart JP.(2005)Involvement of PI3K/Akt pathway in prostate cancer –potential strategies for developing targeted therapies.Mini Rev Med Chem.5(12),1125