-
免疫印迹(WB)
(1).gif)
Western blotting (WB) is used to analyze a target protein in a mixed sample with numerous proteins according to the specificity of antibody against its antigen. With SDS-PAGE’s high resolution and solid-phase immunoassay’s high sensitivity and specificity, western blotting has been one of the most common methods for protein analysis. SAB follows a stringent validation protocol and uses several validation approaches in western blotting to ensure antibody quality and phospho-specificity.
Validation Approaches Include:
Phosphatase treatment
Stimulus or inhibitor treatment
Blocking peptide control
Validation by various cell lines
Phosphatase treatment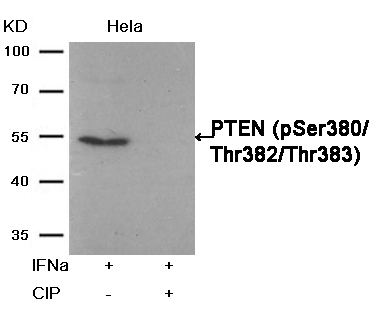 Western blot analysis of extracts from Hela cells, treated with IFNa or calf intestinal phosphatase (CIP), using PTEN (Phospho-Ser380/Thr382/Thr383) Antibody #11056.Stimulus or inhibitor treatment
Western blot analysis of extracts from Hela cells, treated with IFNa or calf intestinal phosphatase (CIP), using PTEN (Phospho-Ser380/Thr382/Thr383) Antibody #11056.Stimulus or inhibitor treatment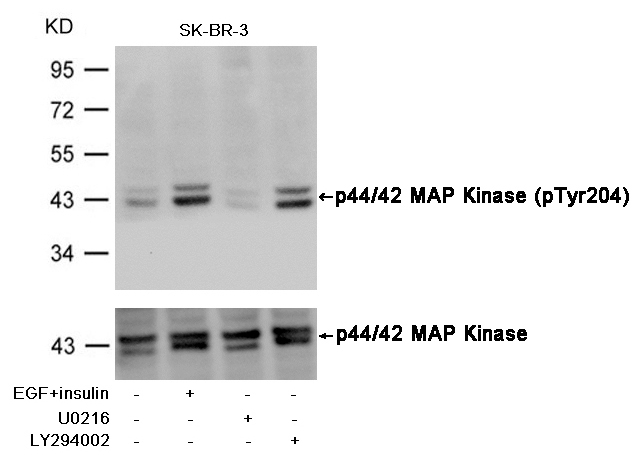 Western blot analysis of extracts from SK-BR-3 cells, untreated or insulin and EGF treated, and pretreated with U0126 and LY294002, using p44/42 MAP Kinase (Phospho-Tyr204) Antibody #11246 (upper) or p44/42 MAP Kinase Antibody #21238 (lower) .Fluorescent Western Blotting
Western blot analysis of extracts from SK-BR-3 cells, untreated or insulin and EGF treated, and pretreated with U0126 and LY294002, using p44/42 MAP Kinase (Phospho-Tyr204) Antibody #11246 (upper) or p44/42 MAP Kinase Antibody #21238 (lower) .Fluorescent Western Blotting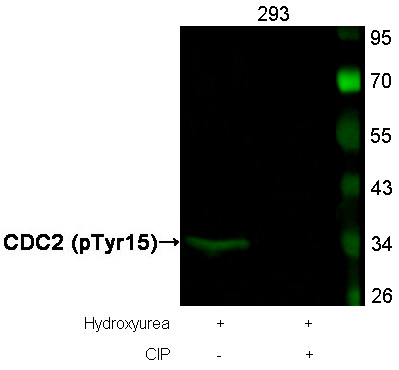 Western blot analysis of extracts from 293 cells, treated with Hydroxyurea or calf intestinal phosphatase(CIP), using CDC2 (Phospho-Tyr15) Antibody . #11244.Blocking peptide control
Western blot analysis of extracts from 293 cells, treated with Hydroxyurea or calf intestinal phosphatase(CIP), using CDC2 (Phospho-Tyr15) Antibody . #11244.Blocking peptide control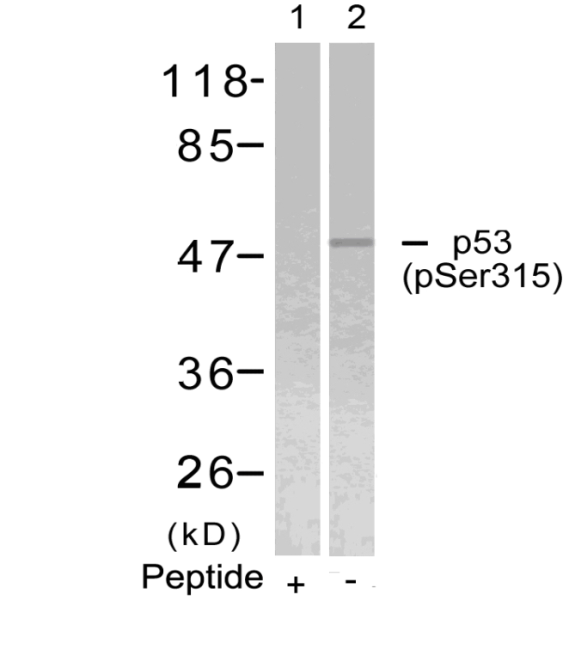 Western blot analysis of extracts from Hela cells using p53 (Phospho-Ser315) Antibody #11100 (Lane 2) and the same antibody preincubated with blocking peptide (Lane1).Validation by various cell lines
Western blot analysis of extracts from Hela cells using p53 (Phospho-Ser315) Antibody #11100 (Lane 2) and the same antibody preincubated with blocking peptide (Lane1).Validation by various cell lines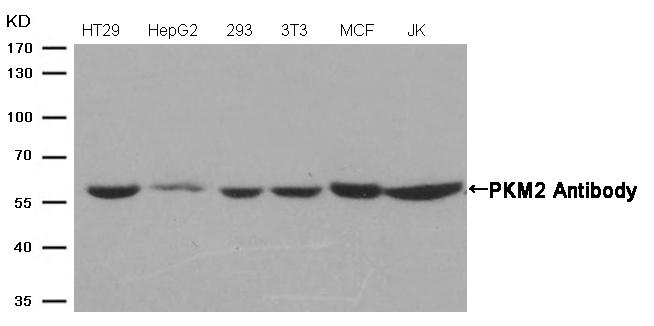 Western blot analysis of extracts from various cells using PKM2 Antibody #21578.
Western blot analysis of extracts from various cells using PKM2 Antibody #21578.

-
利用磷酸酯酶的新磷酸化特异性验证
In order to further verify phospho-specificity and ensure quality, SAB has increased phosphatase treatment method in validation of phospho-specific antibodies. Up to the present SAB has used numerous analytical methods in antibody characterization.
Western blotting(WB)
Phosphatase treatment
Stimulus treatment
Blocking peptide control
Loading control
Validation by various cell lines
Immunohistochemistry(IHC)
Blocking peptide control
Validation with different tissues
Immunofluorescence / Immunocytochemistry(IF/ICC)
Validation by various cell lines
Subcellular localization and co-localization analysis
Flow cytometry(FCM)
ELISA
Akt(Phospho-Ser473) Antibody 11054 Western blot analysis of extracts from 293 cells, treatedwith EGF or calf intestinal phosphatase (CIP), using Akt(Phospho-Ser473) Antibody #11054. MORE> 
PTEN(Phospho-Ser380/Thr382/Thr383) Antibody 11056 Western blot analysis of extracts from Hela cells, treated with IFNa or calf intestinal phosphatase (CIP), using PTEN (Phospho-Ser380/Thr382/Thr383) Antibody #11056. MORE> 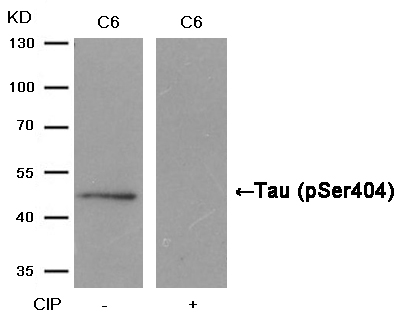
Tau(Phospho-Ser404) Antibody 11112 Western blot analysis of extracts from C6 cells, treated with calf intestinal phosphatase (CIP), using Tau (Phospho-Ser404) Antibody #11112. MORE> 
NFkB-p65(Phospho-Ser529) Antibody 11217 Western blot analysis of extracts from MCF cells, treated with TNFa+CA or calf intestinal phosphatase (CIP), using NFκB-p65 (Phospho-Ser529) Antibody #11217. MORE> 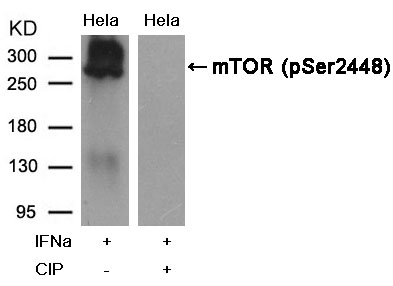
mTOR(Phospho-Ser2448) Antibody 11221 Western blot analysis of extracts from Hela cells, treated with IFNa or calf intestinal phosphatase (CIP), using mTOR (Phospho-Ser2448) Antibody #11221. MORE> 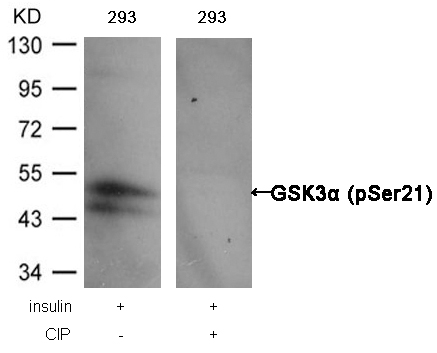
GSK3α (Phospho-Ser21) Antibody 11007 Western blot analysis of extracts from 293 cells, treated with insulin or calf intestinal phosphatase (CIP), using GSK3α (Phospho-Ser21) Antibody #11007. MORE> 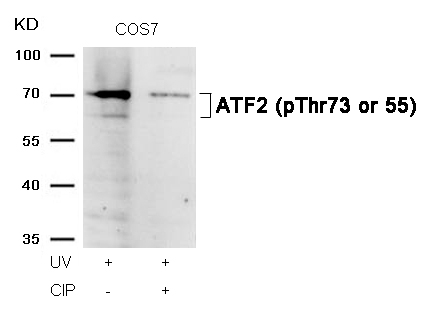
ATF2 (Phospho-Thr73 or 55) Antibody 11032 Western blot analysis of extracts from COS7 cells, treated with UV or calf intestinal phosphatase (CIP), using ATF2 (Phospho-Thr73 or 55) Antibody #11032. MORE> 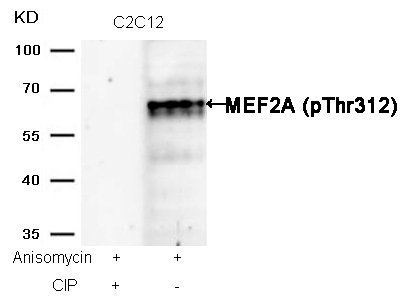
MEF2A (Phospho-Thr312) Antibody 11039 Western blot analysis of extracts from C2C12 cells, treated with Anisomycin or calf intestinal phosphatase (CIP), using MEF2A (Phospho-Thr312) Antibody #11039. MORE> 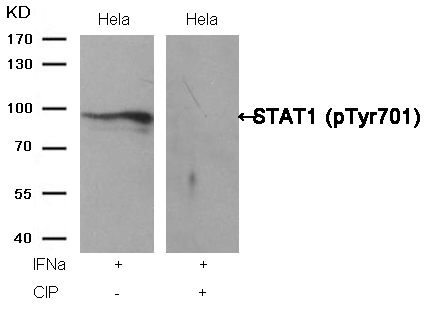
STAT1 (Phospho-Tyr701) Antibody 11044 Western blot analysis of extracts from Hela cells, treated with IFNa or calf intestinal phosphatase (CIP), using STAT1 (Phospho-Tyr701) Antibody #11044. MORE> 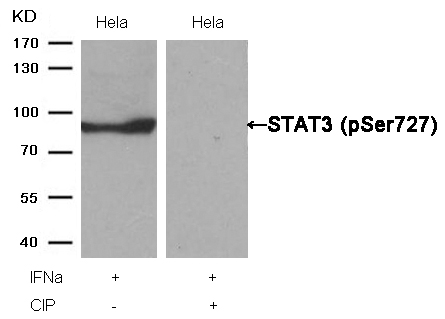
STAT3 (Phospho-Ser727) Antibody 11046 Western blot analysis of extracts from Hela cells, treated with IFNa or calf intestinal phosphatase (CIP), using STAT3 (Phospho-Ser727) Antibody #11046. MORE> 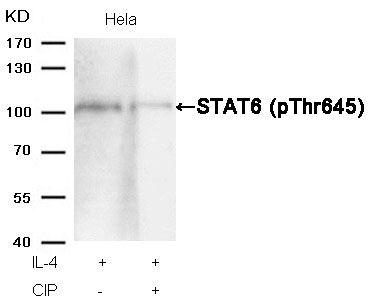
STAT6 (Phospho-Thr645) Antibody 11051 Western blot analysis of extracts from Hela cells, treated with IL-4 or calf intestinal phosphatase (CIP), using STAT6 (Phospho-Thr645) Antibody #11051. MORE> 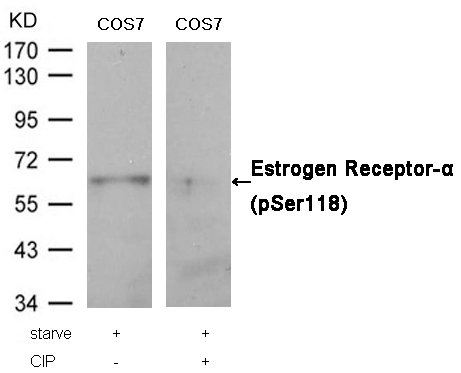
Estrogen Receptor-α (Phospho-Ser118) Antibody 11072 Western blot analysis of extracts from COS7 cells, treated with starve or calf intestinal phosphatase (CIP), using Estrogen Receptor-α (Phospho-Ser118) Antibody #11072. MORE> 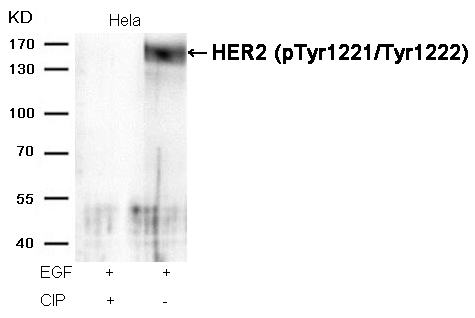
HER2 (Phospho-Tyr1221/Tyr1222) Antibody 11076 Western blot analysis of extracts from Hela cells, treated with EGF or calf intestinal phosphatase (CIP), using HER2 (Phospho-Tyr1221/Tyr1222) Antibody #11076. MORE> 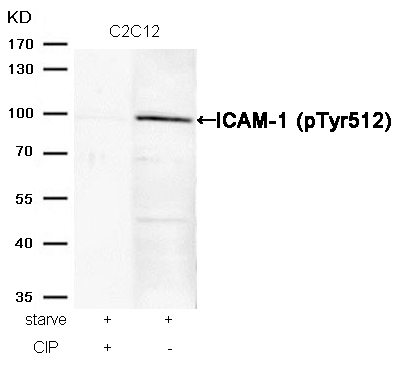
ICAM-1 (Phospho-Tyr512) Antibody 11083 Western blot analysis of extracts from C2C12 cells, treated with starve or calf intestinal phosphatase (CIP), using ICAM-1 (Phospho-Tyr512) Antibody #11083. MORE> 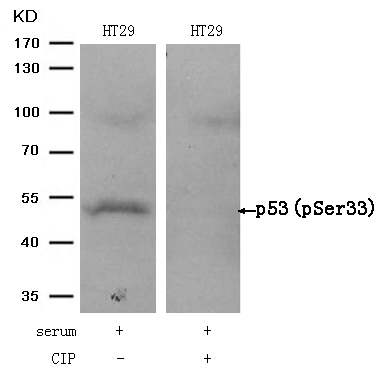
p53 (Phospho-Ser33) Antibody 11097 Western blot analysis of extracts from HT29 cells, treated with serum or calf intestinal phosphatase (CIP), using p53 (Phospho-Ser33) Antibody #11097. MORE> 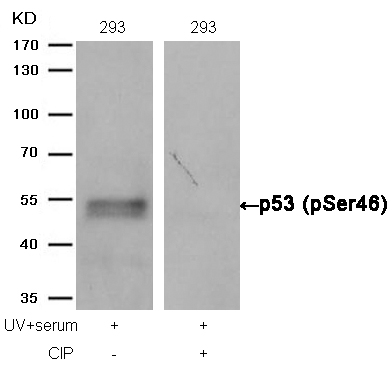
p53 (Phospho-Ser46) Antibody 11099 Western blot analysis of extracts from 293 cells, treated with UV+serum or calf intestinal phosphatase (CIP), using p53 (Phospho-Ser46) Antibody #11099. MORE> 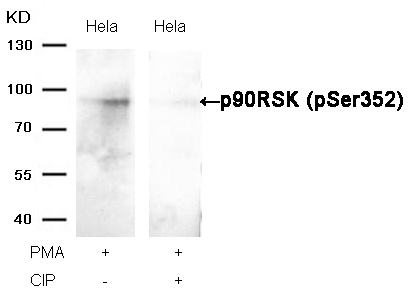
p90RSK (Phospho-Ser352) Antibody 11113 Western blot analysis of extracts from Hela cells, treated with PMA or calf intestinal phosphatase (CIP), using p90RSK (Phospho-Ser352) Antibody #11113. MORE> 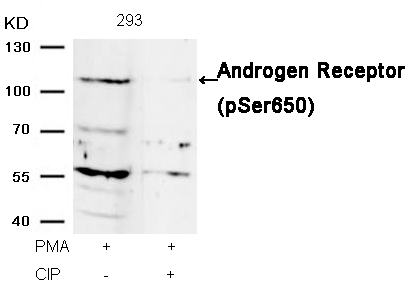
Androgen Receptor (Phospho-Ser650) Antibody 11120 Western blot analysis of extracts from 293 cells, treated with PMA or calf intestinal phosphatase (CIP), using Androgen Receptor (Phospho-Ser650) Antibody #11120. MORE> 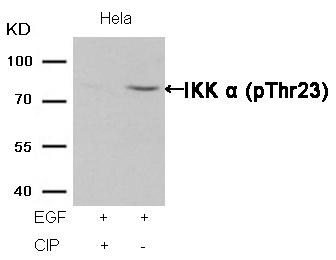
IKK α (Phospho-Thr23) Antibody 11129 Western blot analysis of extracts from Hela cells, treated with EGF or calf intestinal phosphatase (CIP), using IKK α (Phospho-Thr23) Antibody #11129. MORE> 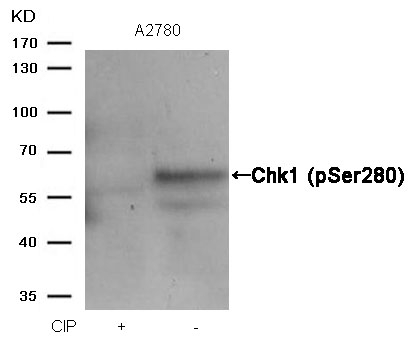
Chk1 (Phospho-Ser280) Antibody 11140 Western blot analysis of extracts from A2780 cells, treated with calf intestinal phosphatase (CIP), using Chk1 (Phospho-Ser280) Antibody #11140. MORE> 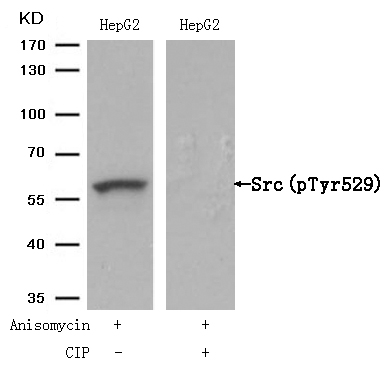
Src(Phospho-Tyr529) Antibody 11153 Western blot analysis of extracts from HepG2 cells, treated with Anisomycin or calf intestinal phosphatase (CIP), using Src (Phospho-Tyr529) Antibody #11153. MORE> 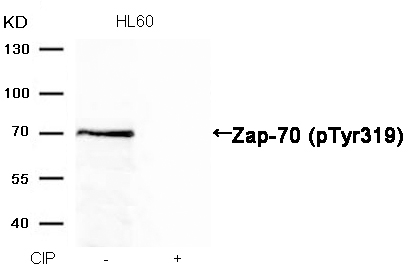
Zap-70 (Phospho-Tyr319) Antibody 11159 Western blot analysis of extracts from HL60 cells, treated with calf intestinal phosphatase (CIP), using Zap-70 (Phospho-Tyr319) Antibody #11159. MORE> 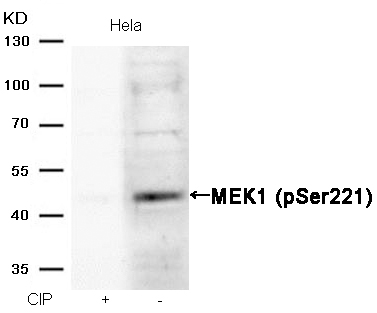
MEK1 (Phospho-Ser221) Antibody 11161 Western blot analysis of extracts from Hela cells, treated with calf intestinal phosphatase (CIP), using MEK1 (Phospho-Ser221) Antibody #11161. MORE> 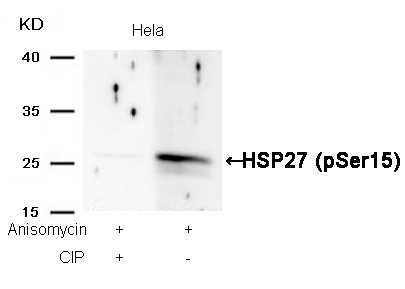
HSP27 (Phospho-Ser15) Antibody 11164 Western blot analysis of extracts from Hela cells, treated with Anisomycin or calf intestinal phosphatase (CIP), using HSP27 (Phospho-Ser15) Antibody #11164. MORE> 
AMPKα1 (Phospho-Ser487)Antibody 11174 Western blot analysis of extracts from C6 cells, treated with EGF or calf intestinal phosphatase (CIP), using AMPKα1 (Phospho-Ser487) Antibody #11174. MORE> 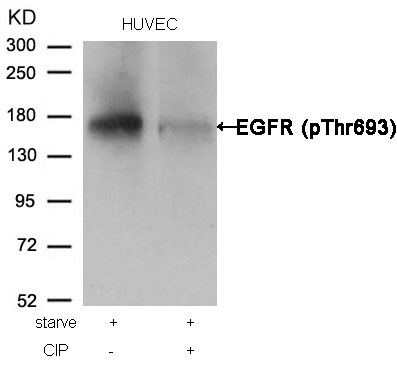
EGFR (Phospho-Thr693) Antibody 11187 Western blot analysis of extracts from HUVEC cells, treated with starve or calf intestinal phosphatase (CIP), using EGFR (Phospho-Thr693) Antibody #11187. MORE> 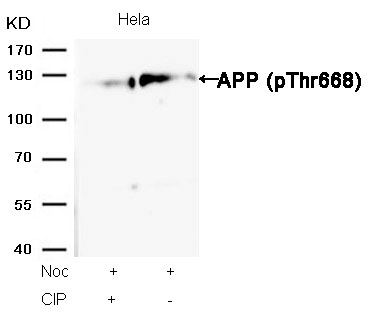
APP (Phospho-Thr668) Antibody 11190 Western blot analysis of extracts from Hela cells, treated with Noc or calf intestinal phosphatase (CIP), using APP (Phospho-Thr668) Antibody #11190. MORE> 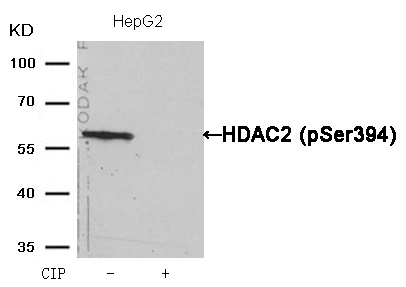
HDAC2 (Phospho-Ser394) Antibody 11191 Western blot analysis of extracts from HepG2 cells, treated with calf intestinal phosphatase (CIP), using HDAC2 (Phospho-Ser394) Antibody #11191. MORE> 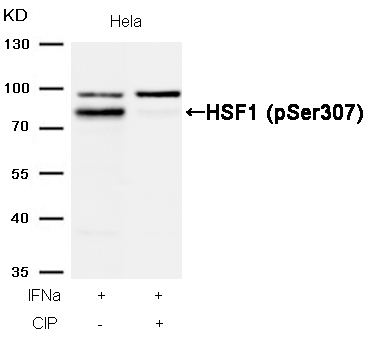
HSF1 (Phospho-Ser307) Antibody 11195 Western blot analysis of extracts from Hela cells, treated with IFNa or calf intestinal phosphatase (CIP), using HSF1 (Phospho-Ser307) Antibody #11195. MORE> 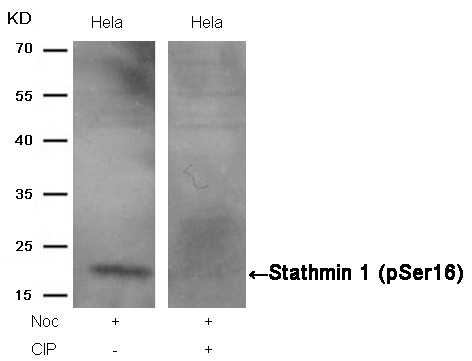
Stathmin 1 (Phospho-Ser16) Antibody 11234 Western blot analysis of extracts from Hela cells, treated with Noc or calf intestinal phosphatase (CIP), using Stathmin 1 (Phospho-Ser16) Antibody #11234. MORE> 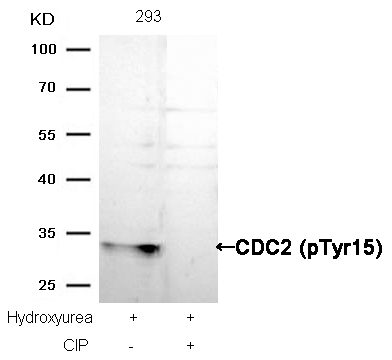
CDC2 (Phospho-Tyr15) Antibody 11244 Western blot analysis of extracts from Hela cells, treated with Noc or calf intestinal phosphatase (CIP), using Stathmin 1 (Phospho-Ser16) Antibody #11244. MORE> 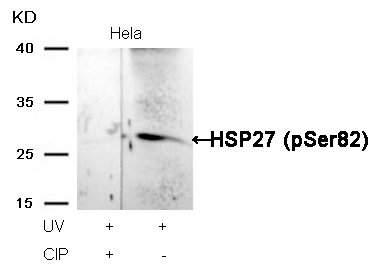
HSP27 (Phospho-Ser82) Antibody 11248 Western blot analysis of extracts from Hela cells, treated with UV or calf intestinal phosphatase (CIP), using HSP27 (Phospho-Ser82) Antibody #11248. MORE> 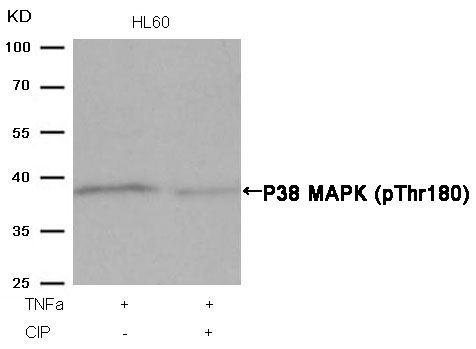
P38 MAPK (Phospho-Thr180) Antibody 11252 Western blot analysis of extracts from HL60 cells, treated with TNFa or calf intestinal phosphatase (CIP), using P38 MAPK (Phospho-Thr180) Antibody #11252. MORE> 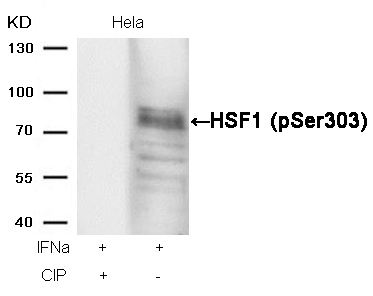
HSF1 (phospho-Ser303) Antibody 11263 Western blot analysis of extracts from Hela cells, treated with IFNa or calf intestinal phosphatase (CIP), using HSF1 (phospho-Ser303) Antibody #11263. MORE> 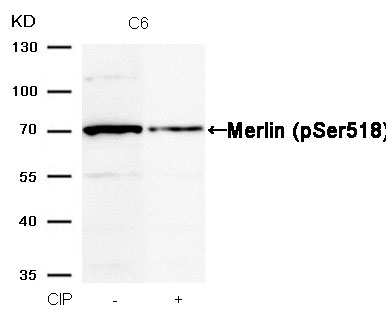
Merlin (Phospho-Ser518) Antibody 11266 Western blot analysis of extracts from C6 cells, treated with calf intestinal phosphatase (CIP), using Merlin (Phospho-Ser518) Antibody #11266. MORE> 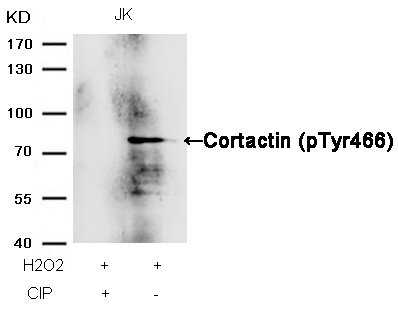
Cortactin (Phospho-Tyr466) Antibody 11272 Western blot analysis of extracts from JK cells, treated with H2O2 or calf intestinal phosphatase (CIP), using Cortactin (Phospho-Tyr466) Antibody #11272. MORE> 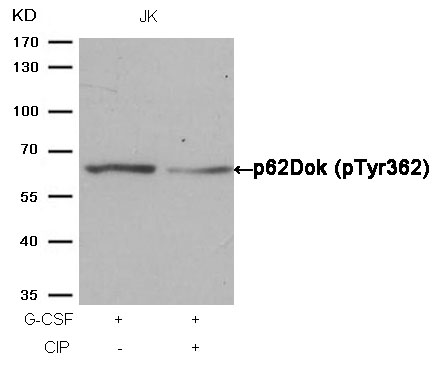
p62Dok (phospho-Tyr362) Antibody 11276 Western blot analysis of extracts from JK cells, treated with G-CSF or calf intestinal phosphatase (CIP), using p62Dok (phospho-Tyr362) Antibody #11276. MORE> 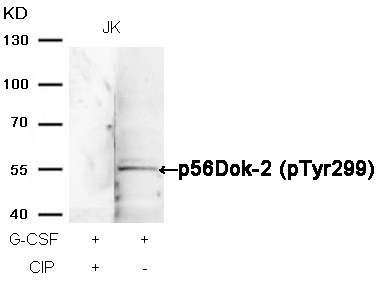
p56Dok-2 (Phospho-Tyr299) Antibody 11278 Western blot analysis of extracts from JK cells, treated with G-CSF or calf intestinal phosphatase (CIP), using p56Dok-2 (Phospho-Tyr299) Antibody #11278. MORE> 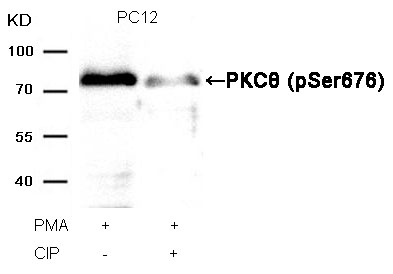
PKCθ (Phospho-Ser676) Antibody 11297 Western blot analysis of extracts from PC12 cells, treated with PMA or calf intestinal phosphatase (CIP), using PKCθ (Phospho-Ser676) Antibody #11297. MORE> 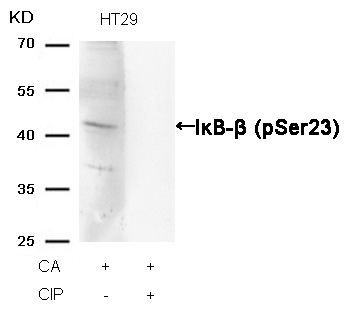
IκB-β (Phospho-Ser23) Antibody 11304 Western blot analysis of extracts from HT29 cells, treated with CA or calf intestinal phosphatase (CIP), using IκB-β (Phospho-Ser23) Antibody #11304. MORE> 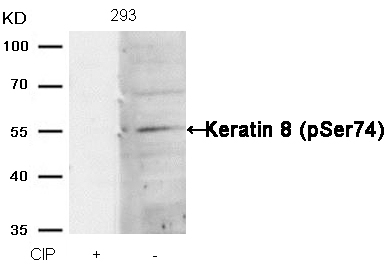
Keratin 8 (Phospho-Ser74) Antibody 11307 Western blot analysis of extracts from 293 cells, treated with calf intestinal phosphatase (CIP), using Keratin 8 (Phospho-Ser74) Antibody #11307. MORE> 
Myc (Phospho-Ser62) Antibody 11311 Western blot analysis of extracts from K562 cells, treated with calf intestinal phosphatase (CIP), using Myc (Phospho-Ser62) Antibody #11311. MORE> 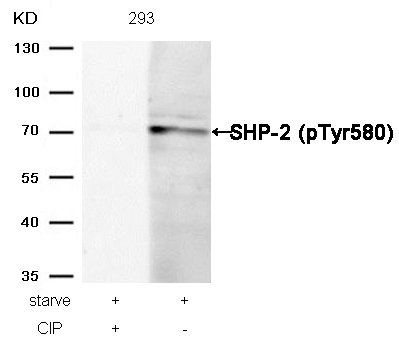
SHP-2 (Phospho-Tyr580) Antibody 11320 Western blot analysis of extracts from 293 cells, treated with starve or calf intestinal phosphatase (CIP), using SHP-2 (Phospho-Tyr580) Antibody #11320. MORE> 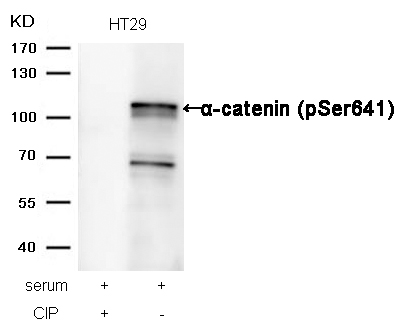
α-catenin (Phospho-Ser641) Antibody 11330 Western blot analysis of extracts from HT29 cells, treated with serum or calf intestinal phosphatase (CIP), using α-catenin (Phospho-Ser641) Antibody #11330. MORE> 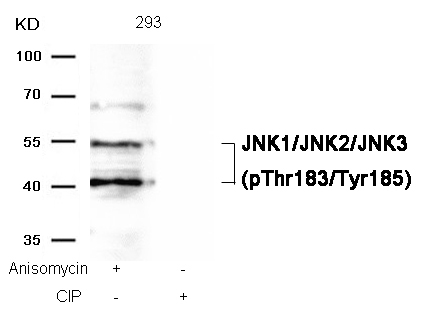
JNK1/JNK2/JNK3 (phospho-Thr183/Tyr185) Antibody 11504 Western blot analysis of extracts from 293 cells, treated with Anisomycin or calf intestinal phosphatase (CIP), using JNK1/JNK2/JNK3 (phospho-Thr183/Tyr185) Antibody #11504. MORE> 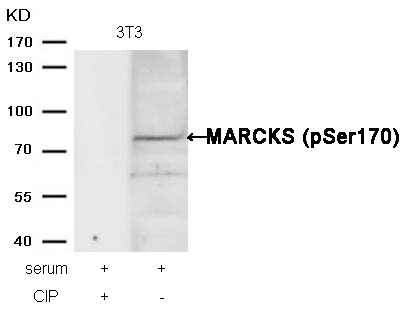
MARCKS (phospho-Ser170) Antibody 11535 Western blot analysis of extracts from 3T3 cells, treated with serum or calf intestinal phosphatase (CIP), using MARCKS (phospho-Ser170) Antibody #11535. MORE> 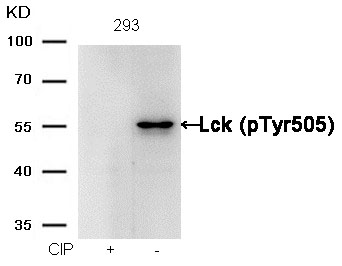
Lck (phospho-Tyr505) Antibody 11537 Western blot analysis of extracts from 293 cells, treated with calf intestinal phosphatase (CIP), using Lck (phospho-Tyr505) Antibody #11537. MORE> 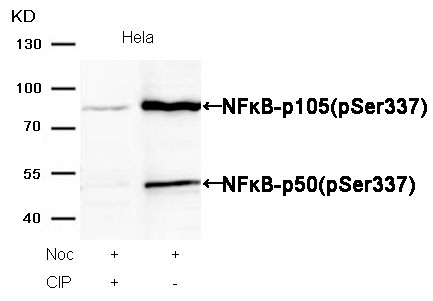
NFκB-p105/p50(Phospho-Ser337) Antibody 11017 Western blot analysis of extracts from Hela cells, treated with Noc or calf intestinal phosphatase (CIP), using NFκB-p105/p50(Phospho-Ser337) Antibody #11017. MORE> -
新发表文献
Volume 35, Issue 1, January 2014, Pages 316–326The use of hollow mesoporous silica nanospheres to encapsulate bortezomib and improve efficacy for non-small cell lung cancer therapyJia Shena, b, c, 1, Guosheng Songd, 1, Man Ana, c, Xianqian Lie, Ning Wue, Kangcheng Ruana, c, Junqing Hud, Ronggui Hua, c,a State Key Laboratory of Molecular Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, 320 Yue-yang Road, Shanghai 200031, Chinab University of Chinese Academy of Sciences, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, 320 Yue-yang Road, Shanghai 200031, Chinac Shanghai Key Laboratory of Molecular Andrology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, 320 Yue-yang Road, Shanghai 200031, Chinad State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, Chinae Department of Clinical Oncology, Xuhui Central Hospital, 966 Middle Huaihai Road, Shanghai 200031, ChinaAbstractBortezomib (BTZ) is the first clinically approved proteasome inhibitor for treating multiple human malignancies. However, the poor water-solubility and low stability of BTZ and the emergence of tumor resistance have severely restrained its therapeutic efficacy. Herein, we report the application of hollow mesoporous silica nanospheres (HMSNs) in encapsulating BTZ for drug delivery. In in vitro cell viability assay on human NSCLC H1299 cells, the half-maximum inhibiting concentration (IC50) of HMSNs–BTZ was 42% of that for free BTZ in 48 h treatments. In vivo tumor-suppression assay further indicated that HMSNs–BTZ (0.3 mg/kg) showed approximately 1.5 folds stronger anti-tumor activity than free BTZ. Furthermore, we report that more potent induction of cell cycle arrest and apoptotic cell death, along with promoted activation of Caspase 3 and autophagy might mechanistically underlie the improved anti-tumor efficacy of HMSNs–BTZ. Finally, the tumor-suppressing effect of HMSNs–BTZ was enhanced in the presence of wild-type p53 signaling, suggesting a potential enhancement in clinical efficacy with combined p53 gene therapy and BTZ-based chemotherapy. Therefore, the HMSNs-based nanoparticles are emerging as a promising platform to deliver therapeutic agents for beneficial clinical outcomes through lowering doses and frequency of drug administration and reducing potential side effects.KeywordsAnti-tumor activity; Apoptotic cell death; Bortezomib; Drug delivery; Mesoporous silica; NSCLC -
发表文献
Cat.No.
Product Name
Reference
11002
GSK3β (Phospho-Ser9) Antibody
Cong REN, Jia-Mou LI, Xin LIN (2010) LIPUS Enhance Elongation of Neurites in Rat Cortical Neurons through Inhibition of GSK-3β. Biomedical and Environmental Sciences, Volume 23, Issue 3, Pages 244-249
11002
GSK3β (Phospho-Ser9) Antibody
Yingjuan Yang, Jinzeng Yang, Rongxin Liu, Huixia Li and Xiao Luo, et al.Accumulation of β-catenin by lithium chloride in porcine myoblast cultures accelerates cell differentiation.Molecular Biology Reports, 2011, Volume 38, Number 3, Pages 2043-2049
11002
GSK3b(Phospho-Ser9) Antibody
Estefanía de Munck, Emma Mu˜noz-Sáez, Bego˜na G. Miguel, M. Teresa Solas,et al (2013) B-N-methylamino-l-alanine causes neurological andpathological phenotypes mimicking Amyotrophic LateralSclerosis (ALS): The first step towards an experimentalmodel for sporadic ALS. environmental toxicology and pharmacology 36:243–255
11002
GSK3b(Phospho-Ser9) Antibody
Yu-fei Pan, Li-wei Dong, Min Wang, Guang-zhen Yang, et al. (2013) Signal regulatory protein α negatively regulates mast-cell activation following FcεRI aggregation. Eur. J. Immunol. 43: 1598–1607
11002
GSK3b(Phospho-Ser9) Antibody
Lei Hana, Yang Yanga, Xiao Yuea, Kai Huanga, et al. (2010) Inactivation of PI3K/AKT signaling inhibits glioma cell growth through modulation of β-catenin-mediated transcription. BRAIN RESEARCH 1366:9–17
11002
GSK3b(Phospho-Ser9) Antibody
Wen-Fei Tan, Xue-Zhao Cao, Jun-KeWang, Huang-Wei Lv, et al. (2010) Protective effects of lithium treatment for spatial memory deficits induced by tau hyperphosphorylation in splenectomized rats. Clinical and Experimental Pharmacology and Physiology. 37:1010–1015
11002
GSK3b(Phospho-Ser9) Antibody
Jiamou Li, Hua Zhang, Cong Ren. (2012) Effect of Low-Intensity Pulsed Ultrasound on Nerve Repair. Tissue Regeneration - From Basic Biology to Clinical Application, Prof. ISBN: 978-953-51-0387-5,
11005
PDK1 (Phospho-Ser241) Antibody
Chao Han, Remi Quirion, Wenhua Zheng et al (2011) Glutamate attenuates IGF-1 receptor signaling via NR2B containing NMDA receptors and neuronal nitric oxide synthase. Biochemical and Biophysical Research Communications, In Press,
11005
PDK1(Phospho-Ser241) Antibody
Dan Liu, Yi Huang, Bojiang Chen, Jing Zeng, et al. (2011) Activation of Mammalian Target of Rapamycin Pathway Confers Adverse Outcome in Nonsmall Cell Lung Carcinoma. DOI: 10.1002/cncr.25959
11006
Raf1(Phospho-Ser259) Antibody
Zhi-Xin Qiu, Lei Wang,Juan Han, Dan Liu, et al.(2012) Prognostic impact of Raf-1 and p-Raf-1 expressions for poor survival rate in non-small cell lung cancer. Cancer Sci. vol. 103 no. 10 pp. 1774–1779
11007
GSK3α (Phospho-Ser21) Antibody
Dan Liu, Yi Huang, Jing Zeng, Bojiang Chen and Na Huang, et al.Down-regulation of JAK1 by RNA interference inhibits growth of the lung cancer cell line A549 and interferes with the PI3K/mTOR pathway. Journal of Cancer Research and Clinical Oncology, 2011, Volume 137, Number 11, Pages 1629-164
11011
NFκB-p65 (Phospho-Ser276) Antibody
Anneleen Spooren, Krzysztof Kolmus, Linda Vermeulen,et al.(2010) Hunting for Serine 276-phosphorylated p65. Journal of Biomedicine and Biotechnology, 2010, Article ID 275892, 9 doi:10.1155/2010/275892
11011
NFκB-p65(Phospho-Ser276) Antibody
Xiaoping Cai, Saul Benedict Freedman, Paul Kenneth Witting.(2013) Serum amyloid A stimulates cultured endothelial cells to migrate and proliferate: inhibition by the multi-kinase inhibitor BIBF1120. doi: 10.1111/1440-1681.12148
11014
NFκB-p65 (Phospho-Ser536) Antibody
Jitakshi De, Robert E. Brown (2010)Tissue-microarray based immunohistochemical analysis of survival pathways in nodular sclerosing classical Hodgkin lymphoma as compared with Non-Hodgkin’s lymphoma. Int J Clin Exp Med,3(1):55-68
11015
NFκB-p100(Phospho-Ser866) Antibody
Chen Shen & Xin-liang Zhao &Weina Ju &Xiao-bing Zou & Li-rong Huo & Wu Yan & Jun-hua Zou &Guo-di Yan & Edmund C. Jenkins &W. Ted Brown &Nanbert Zhong,A Proteomic Investigation of B Lymphocytes in an Autistic Family: A Pilot Study of Exposure to Natural Rubber Latex (NRL) May Lead to Autism,J Mol Neurosci (2011) 43:443–452
11021
c-Jun(Phospho-Thr91) Antibody
Manujendra N. Saha1,2, Hua Jiang3, Yijun Yang1,2, Xiaoyun Zhu1,2, Xiaoming Wang4, Aaron D. Schimmer4, Lugui Qiu5, Hong Chang,Targeting p53 via JNK Pathway: A Novel Role of RITA for Apoptotic Signaling in Multiple Myeloma, January 2012 | Volume 7 | Issue 1 | e30215
11024
c-Jun (Phospho-Thr239) Antibody
Soichiro Yamamura, Kazumori Kawakami, Hiroshi Hirata,et al(2010) Oncogenic Functions of Secreted Frizzled-Related Protein 2 in Human Renal Cancer. Molecular Cancer Therapeurics
11025
c-Jun (Phospho-Ser243) Antibody
Soichiro Yamamura, Kazumori Kawakami, Hiroshi Hirata,et al(2010) Oncogenic Functions of Secreted Frizzled-Related Protein 2 in Human Renal Cancer. Molecular Cancer Therapeurics
11026
JunB(Phospho-Ser79) Antibody
Raffi Vartanian1, Janine Masri, Jheralyn Martin, Cheri Cloninger, et al. (2010) AP-1 Regulates Cyclin D1 and c-MYC Transcription in an AKT-Dependent Manner in Response to mTOR Inhibition: Role of AIP4/Itch-Mediated JUNB Degradation. American Association for Cancer Research. DOI: 10.1158/1541-7786
11027
JunB(Phospho-Ser259) Antibody
Raffi Vartanian1, Janine Masri, Jheralyn Martin, Cheri Cloninger, et al. (2010) AP-1 Regulates Cyclin D1 and c-MYC Transcription in an AKT-Dependent Manner in Response to mTOR Inhibition: Role of AIP4/Itch-Mediated JUNB Degradation. American Association for Cancer Research. DOI: 10.1158/1541-7786
11039
MEF2A (Phospho-Thr312) Antibody
K Satoh, J Ohnishi, A Sato, et al. (2007) Nemo-Like Kinase-Myocyte Enhancer Factor 2A Signaling Regulates Anterior Formation in Xenopus Development.Molecular and Cellular Biology, 27(21):7623-30.
11044
STAT1 (Phospho-Tyr701) Antibody
Jelke J. Fros, Wen Jun Liu, Natalie A. Prow, et al (2010)Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated JAK-STAT signaling. Journal of Virology, 84(20)10877-10887
11044
STAT1 (Phospho-Tyr701) Antibody
Hirokazu Hara, Yoko Nakamura, Masayuki Ninomiya, et al(2011) Inhibitory effects of chalcone glycosides isolated from Brassica rapa L. ‘hidabeni’ and their synthetic derivatives on LPS-induced NO production in microglia. Bioorganic & Medicinal Chemistry, Volume 19, Issue 18, Pages 5559-5568
11044
STAT1(Phospho-Tyr701) Antibody
C.-C.E. Lan, C.-S. Wu, S.-M. Huang, H.-Y. Kuo, et al.(2012) High-glucose environment reduces human b-defensin-2 expression in human keratinocytes: implications for poor diabetic wound healing. British Association of Dermatologists. 166, pp1221–1229
11044
STAT1(Phospho-Tyr701) Antibody
Hsin-Chien Chen,1,2 Hsin-I Ma,1,3 Huey-Kang Sytwu,1,4 Hsing-Won Wang, Chia-Chi V. Chen,5 Shu-Chen Liu,2 Chi-Huang Chen,6 Hang-Kang Chen, and Chih-Hung Wang, Neural Stem Cells Secrete Factors That Promote Auditory Cell Proliferation Via a Leukemia Inhibitory Factor Signaling Pathway,Journal of Neuroscience Research 88:3308–3318 (2010)
11045
STAT3 (Phospho-Tyr705) Antibody
Yuan Guogang,Lu Qian ,Ming Shi (2008) HER2-dependent MMP-7 expression is mediated by activated STAT3.Cellular Signalling, 20:1284–1291
11045
STAT3 (Phospho-Tyr705) Antibody
H Yamaguchi, J Zhu, T Yu, et al. (2006) Low-level bisphenol A increases production of glial fibrillary acidic protein in differentiating astrocyte progenitor cells through excessive STAT3 and Smad1 activation.Toxicology, 226:131-142.
11045
STAT3 (Phospho-Tyr705) Antibody
Yamaguchi, J Zhu, T Yu, et al. (2007) Serum-free mouse embryo cells generate a self-sustaining feedback loop for an astrocyte marker protein and respond to cytokines and bisphenol A in accordance with the subtle difference in their differentiation state.Cell Biology International, 31(6):638-644.
11045
STAT3 (Phospho-Tyr705) Antibody
Li-Nan Ren , Qing-Fang Li , Feng-Jun Xiao, et al (2009)Endocrine glands-derived vascular endothelial growth factor protects pancreatic cancer cells from apoptosis via upregulation of the myeloid cell leukemia-1 protein.Biochemical and Biophysical Research Communications 386:35–39
11045
STAT3 (Phospho-Tyr705) Antibody
Jian-Guo Zhang, Jing Zhao, and Yan Xin, et al (2010) Significance and relationship between Cripto-1 and p-STAT3 expression in gastric cancer and precancerous lesions. World J Gastroenterol. 16(5): 571–577.
11045
STAT3 (Phospho-Tyr705) Antibody
Takuya Takeichi, Kazumitsu Sugiura, Yoshinao Muro, et al (2010)Overexpression of LEDGF/DFS70 Induces IL-6 via p38 Activation in HaCaT Cells, Similar to that Seen in the Psoriatic Condition. Journal of Investigative Dermatology 130, 2760-2767
11045
STAT3 (Phospho-Tyr705) Antibody
Emilio García-Prieto, Adrián González-López,, Sandra Cabrera, et al (2010)Resistance to Bleomycin-Induced Lung Fibrosis in MMP-8 Deficient Mice Is Mediated by Interleukin-10. PloS one, 5(10): e13242.
11045
STAT3(Phospho-Tyr705) Antibody
Libing Ma, Jinxiu Li, Guyi Wang, Subo Gong, et al.(2013) Atrial natriuretic peptide suppresses Th17 development through regulation of cGMP-dependent protein kinase and PI3K–Akt signaling pathways. Regulatory Peptides. 181:9–16
11045
STAT3(Phospho-Tyr705) Antibody
Feng-Ze Wanga,b, Peng-Jiaoc, Na-Na Yangc, Chuang-Yuana, Ya-Li Zhaoa, Qiang-Qiang Liua, Hong-Rong Fei d, Ji-Guo Zhang, PF-04691502 triggers cell cycle arrest, apoptosis and inhibits the angiogenesis in hepatocellular carcinoma cells, Toxicology Letters 220 (2013) 150– 156
11046
STAT3 (Phospho-Ser727) Antibody
H Yamaguchi, J Zhu, T Yu, et al. (2006) Low-level bisphenol A increases production of glial fibrillary acidic protein in differentiating astrocyte progenitor cells through excessive STAT3 and Smad1 activation.Toxicology, 226:131-142.
11046
STAT3 (Phospho-Ser727) Antibody
Yamaguchi, J Zhu, T Yu, et al. (2007) Serum-free mouse embryo cells generate a self-sustaining feedback loop for an astrocyte marker protein and respond to cytokines and bisphenol A in accordance with the subtle difference in their differentiation state.Cell Biology International, 31(6):638-644.
11046
STAT3 (Phospho-Ser727) Antibody
Jian-Guo Zhang, Jing Zhao, and Yan Xin, et al (2010) Significance and relationship between Cripto-1 and p-STAT3 expression in gastric cancer and precancerous lesions. World J Gastroenterol. 16(5): 571–577.
11046
STAT3(Phospho-Ser727) Antibody
ANIRBAN MAJUMDER, SASWATI BANERJEE, JOSHUA A. HARRILL, DAVID W. MACHACEK,et al (2012) Neurotrophic Effects of Leukemia Inhibitory Factor on Neural Cells Derived from Human Embryonic Stem Cells. STEM CELLS. 30:2387–2399
11046
STAT3(Phospho-Ser727) Antibody
Heng-Chao Yu, Hong-Yan Qin, Fei He, Lin Wang, et al. (2011) Canonical Notch Pathway Protects Hepatocytes from Ischemia/Reperfusion Injury in Mice by Repressing Reactive Oxygen Species Production Through JAK2/STAT3 Signaling. HEPATOLOGY, Vol. 54, No. 3, 979-988
11046
STAT3(Phospho-Ser727) Antibody
Feng-Ze Wanga,b, Peng-Jiaoc, Na-Na Yangc, Chuang-Yuana, Ya-Li Zhaoa, Qiang-Qiang Liua, Hong-Rong Fei d, Ji-Guo Zhang, PF-04691502 triggers cell cycle arrest, apoptosis and inhibits the angiogenesis in hepatocellular carcinoma cells, Toxicology Letters 220 (2013) 150– 156
11048
STAT5a(Phospho-Tyr694) Antibody
Drechsler J, Gro¨ tzinger J, Hermanns HM (2012) Characterization of the Rat Oncostatin M Receptor Complex Which Resembles the Human, but Differs from the Murine Cytokine Receptor. PLoS ONE 7(8): e43155. doi:10.1371/journal.pone.0043155
11052
anti-phospho CREB antibody
Tomasz Boczek, Anna Kozaczuk, Bozena Ferenc, Michalina Kosiorek and Slawomir Pikula, et al.Gene expression pattern in PC12 cells with reduced PMCA2 or PMCA3 isoform: selective up-regulation of calmodulin and neuromodulin.Molecular and Cellular Biochemistry, 12 September 2011
11054
Akt (Phospho-Ser473) Antibody
Zhi Wang, Guotong Xu, Yalan Wu, et al. (2007) Neuregulin-1 enhances differentiation of cardiomyocytes from embryonic stem cells.Medical and Biological Engineering and Computing, 47:41–48
11054
Akt (Phospho-Ser473) Antibody
Seyoon Kim, Yong Zu Lee, Yu Sam Kim,et al (2008)A Proteomic approach for protein-profiling the oncogenic ras induced transformation (H-, K-, and N-Ras) in NIH/3T3 mouse embryonic fibroblasts. Proteomics, 8 (15), 3082 - 3093
11054
Akt (Phospho-Ser473) Antibody
Young Yil Bahk, Ick-Hyun Cho, Tong Soo Kim. (2008)A Cross-talk between oncogenic Ras and tumor suppressor PTEN through FAK Tyr861 phosphorylation in NIH/3T3 mouse embryonic fibroblasts,.Biochemical and Biophysical Research Communications, 377:1199–1204.
11054
Akt (Phospho-Ser473) Antibody
Hui Zhou, Jing Zhao, Xujia Zhang. (2009) Inhibition of uncoupling protein 2 by genipin reduces insulin-stimulated glucose uptake in 3T3-L1 adipocytesArchives of Biochemistry and Biophysics, 486:88–93
11054
Akt (Phospho-Ser473) Antibody
Jing Zhang, Osamu Yamada, Yoshihisa Matsushita, ect.(2009)Transactivation of human osteopontin promoter by human T-cell leukemia virus type 1-encoded Tax protein Leukemia Research in press.
11054
Akt (Phospho-Ser473) Antibody
Li-Nan Ren , Qing-Fang Li , Feng-Jun Xiao, et al (2009)Endocrine glands-derived vascular endothelial growth factor protects pancreatic cancer cells from apoptosis via upregulation of the myeloid cell leukemia-1 protein.Biochemical and Biophysical Research Communications 386 (2009) 35–39
11054
Akt(Phospho-Ser473) Antibody
Ning LI, Geng-tao LIU. (2010) The novel squamosamide derivative FLZ enhances BDNF/TrkB/CREB signaling and inhibits neuronal apoptosis in APP/PS1 mice. Acta Pharmacologica Sinica. 31: 265–272
11054
Akt (Phospho-Ser473) Antibody
Sheng-Li Lin , Li-Ying Yan , Xin-Tian Zhang ,et al.(2010)ER-a36, a Variant of ER-a, Promotes Tamoxifen Agonist Action in Endometrial Cancer Cells via the MAPK/ERK and PI3K/Akt Pathways. PLoS ONE 5(2): e9013. doi:10.1371/journal.pone.0009013
11054
Akt (Phospho-Ser473) Antibody
Chao Han, Remi Quirion, Wenhua Zheng et al (2011) Glutamate attenuates IGF-1 receptor signaling via NR2B containing NMDA receptors and neuronal nitric oxide synthase. Biochemical and Biophysical Research Communications, In Press
11054
Akt(Phospho-Ser473) Antibody
Heng-Chao Yu, Hong-Yan Qin, Fei He, Lin Wang, et al. (2011) Canonical Notch Pathway Protects Hepatocytes from Ischemia/Reperfusion Injury in Mice by Repressing Reactive Oxygen Species Production Through JAK2/STAT3 Signaling. HEPATOLOGY, Vol. 54, No. 3, 979-988
11054
Akt(Phospho-Ser473) Antibody
Hu Ma, Quan Yao, An-Mei Zhang, Sheng Lin, Xin-Xin Wang, Lei Wu, Jian-Guo Sun,and Zheng-Tang Chen(2011)The Effects of Artesunate on the Expression of EGFR and ABCG2 in A549 Human Lung Cancer Cells and a Xenograft Model. Molecules 2011, 16, 10556-10569; doi:10.3390/molecules161210556
11054
Akt(Phospho-Ser473) Antibody
Marc Vendrell, Anabel Molero, Sergio Gonzalez, Kamil Perez-Capote, et al. (2011) Biotin Ergopeptide Probes for Dopamine Receptors. J. Med. Chem. 54, 1080–1090
11054
Akt (Phospho-Ser473) Antibody
Yingjia Guo, Tong Yang, Jun Lu, et al (2011) Rb1 postconditioning attenuates liver warm ischemia–reperfusion injury through ROS-NO-HIF pathway. Life Sciences, Volume 88, Issues 13-14, Pages 598-605
11054
Akt(Phospho-Ser473) Antibody
Lucía Callén, Estefanía Moreno, Pedro Barroso-Chinea, David Moreno-Delgado, Antoni Cortés,et al (2012) Cannabinoid Receptors CB1 and CB2 Form Functional Heteromers in Brain. THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 287, NO. 25, pp. 20851–20865,
11054
Akt(Phospho-Ser473) Antibody
Fang Wang a,b,1, Ting Li a,1, Bin Zhang c,1, Hong Li a, Qiong Wua, Li Yang b,⇑, Yongzhan Nie a, Kaichun Wua, Yongquan Shi a,⇑, Daiming Fan,Biochemical and Biophysical Research Communications, Biochemical and Biophysical Research Communications 434 (2013) 688–694
11054
anti-phospho-STAT3
Heng-Fei Luan1*, Zhi-Bin Zhao1*, Qi-Hong Zhao2, Pin Zhu1, Ming-Yu Xiu1 and Yong Ji, Hydrogen sulfide postconditioning protects isolated rat hearts against ischemia and reperfusion injury mediated by the JAK2/STAT3 survival pathway
11054
Akt(Phospho-Ser473) Antibody
Hongxia Zhanga,1, Junjie Houb,1, Ruina Cuia, Xuejiang Guoc, Zhimin Shia, Fuquan Yangb, Jiayin Daia, Phosphoproteome analysis reveals an important role for glycogen synthase kinase-3 in perfluorododecanoic acid-induced rat liver toxicity,Toxicology Letters 218 (2013) 61– 69
11054
Akt(Phospho-Ser473) Antibody
Jia J, Xu X, Liu F, Guo X, Zhang M, et al. (2013) Identification, Design and Bio-Evaluation of Novel Hsp90 Inhibitors by Ligand-Based Virtual Screening. PLoS ONE 8(4): e59315. doi:10.1371/journal.pone.0059315
11054
Akt(Phospho-Ser473) Antibody
Libing Ma, Jinxiu Li, Guyi Wang, Subo Gong, et al.(2013) Atrial natriuretic peptide suppresses Th17 development through regulation of cGMP-dependent protein kinase and PI3K–Akt signaling pathways. Regulatory Peptides. 181:9–16
11054
Akt(Phospho-Ser473) Antibody
Peter Schubert, Danielle Coupland, Brankica Culibrk, Raymond P. Goodrich, et al. (2013) Riboflavin and ultraviolet light treatment of platelets triggers p38MAPK signaling: inhibition significantly improves in vitro platelet quality after pathogen reduction treatment. TRANSFUSION. Volume **, ** **
11054
Akt(Phospho-Ser473) Antibody
Yunye Ning, Haidong Huang, Yuchao Dong, Qinying Sun,ett al. (2013) 5-Aza-20-deoxycytidine inhibited PDGF-induced rat airway smooth muscle cell phenotypic switching. Arch Toxicol 87:871–881
11054
Akt(Phospho-Ser473) Antibody
Shen J, et al., The use of hollow mesoporous silica nanospheres to encapsulate bortezomib and improve efficacy for non-small cell lung cancer therapy, Biomaterials (2013) Volume 35, Issue 1, January 2014, Pages 316–326
11055
Akt (Phospho-Thr308) Antibody
Alexandra V. Andreeva, Jingyan Han, Mikhail A. Kutuzov ,et al.(2010)Journal of cellular Physiology, 223(1) 1, 94 - 102 T-cadherin modulates endothelial barrier function.
11055
Akt (Phospho-Thr308) Antibody
Chao Han, Remi Quirion, Wenhua Zheng et al (2011) Glutamate attenuates IGF-1 receptor signaling via NR2B containing NMDA receptors and neuronal nitric oxide synthase. Biochemical and Biophysical Research Communications, In Press
11055
Akt(Phospho-Thr308) Antibody
Yingjia Guo, Tong Yang, Jun Lu, Shengfu Li, et al. (2011) Rb1 postconditioning attenuates liver warm ischemia–reperfusion injury through ROS-NO-HIF pathway. Life Sciences. 88:598–605
11055
Akt(Phospho-Thr308) Antibody
Yunye Ning, Haidong Huang, Yuchao Dong, Qinying Sun,ett al. (2013) 5-Aza-20-deoxycytidine inhibited PDGF-induced rat airway smooth muscle cell phenotypic switching. Arch Toxicol 87:871–881
11055
Akt(Phospho-Thr308) Antibody
Nan Li, Heng Lu, Chunyan Chen, Xiaodong Bu, et al. (2013) Loss of fatty acid synthase inhibits the “HER2-PI3K/Akt axis” activity and malignant phenotype of Caco-2 cells Lipids in Health and Disease. 12:83
11055
Akt(Phospho-Thr308) Antibody
Hongxia Zhanga,1, Junjie Houb,1, Ruina Cuia, Xuejiang Guoc, Zhimin Shia, Fuquan Yangb, Jiayin Daia, Phosphoproteome analysis reveals an important role for glycogen synthase kinase-3 in perfluorododecanoic acid-induced rat liver toxicity,Toxicology Letters 218 (2013) 61– 69
11057
p95/NBS1 (Phospho-Ser343) Antibody
F Carrillo, SA Schneider, AMR Taylor, et al (2009) Prominent Oromandibular Dystonia and Pharyngeal Telangiectasia in Atypical Ataxia Telangiectasia. Cerebellum, 8:22–27
11057
p95/NBS1(Phospho-Ser343) Antibody
Rachid Drissi, Jing Wu, Yafang Hu, Carol Bockhold, and Jeffrey S. Dome(2011)Cancer Prevention Research, 4(12) December 2011
11059
FAK (Phospho-Tyr861) Antibody
Liang Wu , Lei Zhu , Wei-Hao Shi , et al. (2008) Zoledronate inhibits the proliferation,adhesion and migration of vascular smooth muscle cells.European Journal of Pharmacology, 602, 124–131
11059
FAK (Phospho-Tyr861) Antibody
Baiyang Sheng, Bo Song, Zhenhuan Zheng, (2009) Abnormal cleavage of APP impairs its functions in cell adhesion and migration.Neuroscience Letters, 450, 327–33
11059
FAK (Phospho-Tyr861) Antibody
Z Zheng, Y Wei, et al.( 2008) Surface Characterization and Cytocompatibility of Three Chitosan/Polycation Composite Membranes for Guided Bone Regeneration.Journal of Biomaterials Applications,24:209-229
11059
FAK(Phospho-Tyr861) Antibody
Masahiko Kanehira, Toshiaki Kikuchi, Shinya Ohkouchi, Taizou Shibahara, et al. (2012) Targeting Lysophosphatidic Acid Signaling Retards Culture-Associated Senescence of Human Marrow Stromal Cells.PLoS ONE 7(2): e32185. doi:10.1371/journal.pone.0032185
11062
PTEN(Phospho-Ser370) Antibody
Zhi Li & Gong Xiang Liu & Yu Lan Liu & Xi Chen & Xiao Li Huang & Hua Tian Gan, Effect of adenovirus-mediated PTEN gene on ulcerative colitis-associated colorectal cancer, Int J Colorectal Dis (2013) 28:1107–1115
11063
Ezrin (Phospho-Tyr353) Antibody
Yazhou Cui, Tianliang Li, Denglu Zhang,et al.(2010)Expression of Ezrin and Phosphorylated Ezrin (pEzrin) in Pancreatic Ductal Adenocarcinoma. Cancer Investigation 28(3) 242-247
11063
Ezrin(Phospho-Tyr353) Antibody
Yasunori Oda MD, Shinichi Aishima PhD, Katsuya Morimatsu PhD,Akifumi Hayashi PhD, et al.(2012) Differential ezrin and phosphorylated ezrin expression profiles between pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasm, and invasive ductal carcinoma of the pancreas. Human Pathology 44:1487–1498
11066
p-Bcl-xl (Ser62)
Dan Liu, Yi Huang, Jing Zeng, Bojiang Chen and Na Huang, et al.Down-regulation of JAK1 by RNA interference inhibits growth of the lung cancer cell line A549 and interferes with the PI3K/mTOR pathway. Journal of Cancer Research and Clinical Oncology, 2011, Volume 137, Number 11, Pages 1629-164
11068
BAD (Phospho-Ser136) Antibody
N Matsuda, Y Takano, S Kageyama, et al.(2007) Silencing of caspase-8 and caspase-3 by RNA interference prevents vascular endothelial cell injury in mice with endotoxic shock.Cardiovascular Research,76:132-140.
11068
BAD (Phospho-Ser136) Antibody
S. Otsuki, K. Sugiyama, O. Amano, T. Yasui, H. Sakagami (2011) Negative regulation of NaF-induced apoptosis by Bad–CAII complex. Toxicology, Volume 287, Issues 1-3,Pages 131-136
11073
Estrogen Receptor-α (Phospho-Ser167) Antibody
Kazuyoshi Motomura, Makoto Ishitobi, Yoshifumi Komoike, et al (2010) Expression of Estrogen Receptor Beta and Phosphorylation of Estrogen Receptor Alpha Serine 167 Correlate with Progression-Free Survival in Patients with Metastatic Breast Cancer Treated with Aromatase Inhibitors. Oncology,79,1-2
11075
HER2(Phospho-Tyr877) Antibody
M.Alicia Corte´s1, Ariel E.Cariaga-Martinez1,Marı´a V.T.Lobo2,3, Rosa M.Martı´n Orozco1, Omar Motin˜o1,F.Javier Rodrı´guez-Ubreva1,4, Javier Angulo5,Pilar Lo´pez-Ruiz1 and Begon˜a Cola´s1,EGF promotes neuroendocrine-like differentiation of prostate cancer cells in the presence of LY294002 through increased ErbB2 expression independent of the phosphatidylinositol 3-kinase-AKT pathway,Carcinogenesis vol.33 no.6 pp.1169–1177, 2012
11079
HER2 (Phospho-Tyr1248) Antibody
Yuan Guogang,Lu Qian ,Ming Shi (2008)HER2-dependent MMP-7 expression is mediated by activated STAT3Cellular Signalling, 20:1284–1291
11088
anti-phospho-IGF-1R (1165/1166)
Chao Han, Remi Quirion, Wenhua Zheng et al (2011) Glutamate attenuates IGF-1 receptor signaling via NR2B containing NMDA receptors and neuronal nitric oxide synthase. Biochemical and Biophysical Research Communications, In Press,
11090
Caveolin-1 (Phospho-Tyr14) Antibody
André Bento-Abreu, Ana Velasco, Erica Polo-Hernández,et al(2009)Albumin endocytosis via megalin in astrocytes is caveola- and Dab-1 dependent and is required for the synthesis of the neurotrophic factor oleic acid. Journal of Neurochemistry,111 (1), Pages 49 - 60
11092
p53(Phospho-Ser6) Antibody
Yan-Qing Guan a,1, Zhibin Li a,1, Aini Yang a, Zheng Huang a, Zhe Zheng a, Lin Zhang a, Ling Li a, Jun-Ming Liu, Cell cycle arrest and apoptosis of OVCAR-3 and MCF-7 cells induced by co-immobilized TNF-a plus IFN-g on polystyrene and the role of p53 activation, Contents lists available at SciVerse ScienceDirect
11098
p53(Phospho-Ser37) Antibody
Yan-Qing Guan a,1, Zhibin Li a,1, Aini Yang a, Zheng Huang a, Zhe Zheng a, Lin Zhang a, Ling Li a, Jun-Ming Liu, Cell cycle arrest and apoptosis of OVCAR-3 and MCF-7 cells induced by co-immobilized TNF-a plus IFN-g on polystyrene and the role of p53 activation, Contents lists available at SciVerse ScienceDirect
11100
p53(Phospho-Ser315) Antibody
Yan-Qing Guan a,1, Zhibin Li a,1, Aini Yang a, Zheng Huang a, Zhe Zheng a, Lin Zhang a, Ling Li a, Jun-Ming Liu, Cell cycle arrest and apoptosis of OVCAR-3 and MCF-7 cells induced by co-immobilized TNF-a plus IFN-g on polystyrene and the role of p53 activation, Contents lists available at SciVerse ScienceDirect
11102
Tau(Phospho-Ser396) Antibody
Xu-Ying Sun, Yu-Ping Wei, Yan Xiong, Xiao-Chuan Wang, et al. (2012) Synaptic Released Zinc Promotes Tau Hyperphosphorylation by Inhibition of Protein Phosphatase 2A (PP2A). THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 287, NO. 14, pp. 11174–11182
11102
Tau(Phospho-Ser396) Antibody
Li-Ming Chen, Yan-Si Xiong, Fan-Li Kong, Min Qu, et al. (2012) Neurolobin attenuates Alzheimer-like tau hyperphosphorylation by activating Akt signaling. JOURNAL OF NEUROCHEMISTRY. 120:157–164
11102
Tau(Phospho-Ser396) Antibody
Wen-Fei Tan, Xue-Zhao Cao, Jun-KeWang, Huang-Wei Lv, et al. (2010) Protective effects of lithium treatment for spatial memory deficits induced by tau hyperphosphorylation in splenectomized rats. Clinical and Experimental Pharmacology and Physiology. 37:1010–1015
11103
PLCγ1 (Phospho-Tyr783) Antibody
Cheng-Ying Hsieh, Chien-Liang Liu, Ming-Jen Hsu, Thanasekaran Jayakumar, et al. (2010) Inhibition of vascular smooth muscle cell proliferation by the vitamin E derivative pentamethylhydroxychromane in an in vitro and in vivo study: pivotal role of hydroxyl radical-mediated PLCγ1 and JAK2 phosphorylation. Free Radical Biology & Medicine. 49:881–893
11103
PLCgamma1(Phospho-Tyr783) Antibody
Yu-fei Pan, Li-wei Dong, Min Wang, Guang-zhen Yang, et al. (2013) Signal regulatory protein α negatively regulates mast-cell activation following FcεRI aggregation. Eur. J. Immunol. 43: 1598–1607
11106
Tau(Phospho-Ser235) Antibody
Johanne Bertrand, Patrick Senechal, Mathieu Zummo-Soucy, Vanessa Plouffe, et al. (2010) The formation of tau pathological pathological phospho-epitopes in the axon is prevented by the dephosphorylation of selective sites in prinmary hippocampal neurons over-expressing human tau. JOURNAL OF NEUROCHEMISTRY. 114:1353–1367
11107
Tau(Phospho-Thr181) Antibody
Lara Ordóñez-Gutiérrez, Juan María Torres, Rosalina Gavín, Marta Antón, et al. (2013) Cellular prion protein modulates b-amyloid deposition in aged APP/PS1 transgenic mice. Neurobiology of Aging. xxx:1-12
11108
Tau(Phospho-Thr205) Antibody
Tan Wenfei, Cao Xuezhao,Wang Junke,et al. (2010)Tau hyperphosphorylation is associated with memory impairment after exposure to 1.5% isoflurane without temperature maintenance in rats.European Journal of Anaesthesiology, doi: 10.1097/EJA.0b013e32833a6561
11108
Tau(Phospho-Thr205) Antibody
Xu-Ying Sun, Yu-Ping Wei, Yan Xiong, Xiao-Chuan Wang, et al. (2012) Synaptic Released Zinc Promotes Tau Hyperphosphorylation by Inhibition of Protein Phosphatase 2A (PP2A). THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 287, NO. 14, pp. 11174–11182
11108
Tau(Phospho-Thr205) Antibody
Li-Ming Chen, Yan-Si Xiong, Fan-Li Kong, Min Qu, et al. (2012) Neurolobin attenuates Alzheimer-like tau hyperphosphorylation by activating Akt signaling. JOURNAL OF NEUROCHEMISTRY. 120:157–164
11108
Tau(Phospho-Thr205) Antibody
Wen-Fei Tan, Xue-Zhao Cao, Jun-KeWang, Huang-Wei Lv, et al. (2010) Protective effects of lithium treatment for spatial memory deficits induced
by tau hyperphosphorylation in splenectomized rats. Clinical and Experimental Pharmacology and Physiology. 37:1010–101511110
Tau(Phospho-Thr231) Antibody
Xu-Ying Sun, Yu-Ping Wei, Yan Xiong, Xiao-Chuan Wang, et al. (2012) Synaptic Released Zinc Promotes Tau Hyperphosphorylation by Inhibition of Protein Phosphatase 2A (PP2A). THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 287, NO. 14, pp. 11174–11182
11110
Tau(Phospho-Thr231) Antibody
Li-Ming Chen, Yan-Si Xiong, Fan-Li Kong, Min Qu, et al. (2012) Neurolobin attenuates Alzheimer-like tau hyperphosphorylation by activating Akt signaling. JOURNAL OF NEUROCHEMISTRY. 120:157–164
11111
Tau(Phospho-Ser262)Antibody
J. Bertrand, V. Plouffe, P. Sénéchal, N. Leclerc, et al(2010) The pattern of human tau phosphorylation is the result of priming and feedback events in primary hippocampal neurons. Neuroscience, Volume 168, Issue 2, Pages 323-334
11111
Tau(Phospho-Ser262) Antibody
Vanessa Plouffe. (2011) The secretion of the Tau protein: a new mechanism propagation of the pathology of Tau in the disease Alzheimer. University of Montreal Faculty of Graduate and Postdoctoral Studies.
11112
Tau(Phospho-Ser404) Antibody
Xu-Ying Sun, Yu-Ping Wei, Yan Xiong, Xiao-Chuan Wang, et al. (2012) Synaptic Released Zinc Promotes Tau Hyperphosphorylation by Inhibition of Protein Phosphatase 2A (PP2A). THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 287, NO. 14, pp. 11174–11182
11112
Tau(Phospho-Ser404) Antibody
Li-Ming Chen, Yan-Si Xiong, Fan-Li Kong, Min Qu, et al. (2012) Neurolobin attenuates Alzheimer-like tau hyperphosphorylation by activating Akt signaling. JOURNAL OF NEUROCHEMISTRY. 120:157–164
11115
FKHR (Phospho-Ser256) Antibody
Zhan Lixuan ,Tao Wang,Wen Li ,et al.(2010)Journal of Neurochemistry doi: 10.1111/j.1471-4159.2010.06816.xActivation of AKT/FoxO signaling pathway contributes to induction of neuroprotection against transient global cerebral ischemia by hipoxic pre-conditioning in adult rats.
11115
FKHR(Phospho-Ser256) Antibody
Libing Ma, Jinxiu Li, Guyi Wang, Subo Gong, et al.(2013) Atrial natriuretic peptide suppresses Th17 development through regulation of cGMP-dependent protein kinase and PI3K–Akt signaling pathways. Regulatory Peptides. 181:9–16
11122
ATM (Phospho-Ser1981) Antibody
Bin Kang ,Ruifang Guo,Xiao-hui Tian , et al.(2008) Expression status of ataxia telangiectasia mutated gene coorelated with Prognosis in advanced gastric cancer.Mutation Research, 638: 17-25
11122
ATM (Phospho-Ser1981) Antibody
合作伙伴资源
癌症相关信号通路
Akt PathwayApoptosis PathwayCell Cycle RegulationChannel PathwayChromatin/Transcription RegulationCytoskeleton/AdhesionDNA Damage/RepairImmune System RegulationInsulin/Glucose MetabolismJak/Stat PathwayKinases/PhosphatasesMAPK PathwayNF-kappa B PathwayRTKs/AdaptorsStem Cell RegulationTGFb/smads PathwayTranslation RegulationWnt/Notch/Hedgehog Pathway2013年SAB目录
癌基因/抑癌基因蛋白
 An oncogene is a gene that has the potential to cause cancer.[ In tumor cells, they are often mutated or expressed at high levels. Most normal cells undergo a programmed form of death (apoptosis). Activated oncogenes can cause those cells that ought to die to survive and proliferate instead. Most oncogenes require an additional step, such as mutations in another gene, or environmental factors, such as viral infection, to cause cancer. Since the 1970s, dozens of oncogenes have been identified in human cancer. Many cancer drugs target the proteins encoded by oncogenes.A proto-oncogene is a normal gene that can become an oncogene due to mutations or increased expression. The resultant protein may be termed an oncoprotein. Proto-oncogenes code for proteins that help to regulate cell growth and differentiation. Proto-oncogenes are often involved in signal transduction and execution of mitogenic signals, usually through their protein products.Suppressor gene, or anti-oncogene, is a gene that protects a cell from one step on the path to cancer. When this gene is mutated to cause a loss or reduction in its function, the cell can progress to cancer, usually in combination with other genetic changes. Tumor-suppressor genes either have a dampening or repressive effect on the regulation of the cell cycle or promote apoptosis, and sometimes do both.Selected Reviews:Croce CM (2008) Oncogenes and cancer. N Engl J Med. 358 (5): 502-11Yokota J (2000) Tumor progression and metastasis. Carcinogenesis. 21 (3): 497-503May P (1999) Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene. 18(53):7621-36Melamud A (2006) Retinoblastoma. Am Fam Physician 73 (6): 1039-44
An oncogene is a gene that has the potential to cause cancer.[ In tumor cells, they are often mutated or expressed at high levels. Most normal cells undergo a programmed form of death (apoptosis). Activated oncogenes can cause those cells that ought to die to survive and proliferate instead. Most oncogenes require an additional step, such as mutations in another gene, or environmental factors, such as viral infection, to cause cancer. Since the 1970s, dozens of oncogenes have been identified in human cancer. Many cancer drugs target the proteins encoded by oncogenes.A proto-oncogene is a normal gene that can become an oncogene due to mutations or increased expression. The resultant protein may be termed an oncoprotein. Proto-oncogenes code for proteins that help to regulate cell growth and differentiation. Proto-oncogenes are often involved in signal transduction and execution of mitogenic signals, usually through their protein products.Suppressor gene, or anti-oncogene, is a gene that protects a cell from one step on the path to cancer. When this gene is mutated to cause a loss or reduction in its function, the cell can progress to cancer, usually in combination with other genetic changes. Tumor-suppressor genes either have a dampening or repressive effect on the regulation of the cell cycle or promote apoptosis, and sometimes do both.Selected Reviews:Croce CM (2008) Oncogenes and cancer. N Engl J Med. 358 (5): 502-11Yokota J (2000) Tumor progression and metastasis. Carcinogenesis. 21 (3): 497-503May P (1999) Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene. 18(53):7621-36Melamud A (2006) Retinoblastoma. Am Fam Physician 73 (6): 1039-44细胞周期
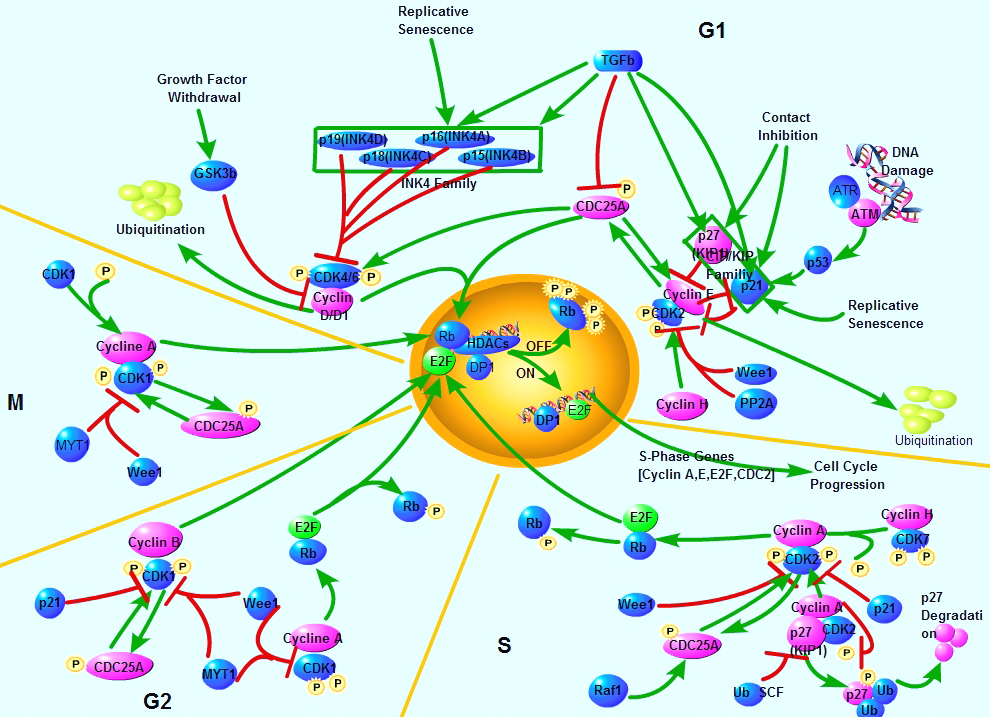 Cell cycle, or cell-division cycle, is the series of events that take place in a cell leading to its division and duplication (replication). Cell cycle control affects many aspects of development. Caenorhabditis elegans cell-cycle genes have been identified over the past decade, including at least two distinct Cyclin-Dependent Kinases (CDKs), their cyclin partners, positive and negative regulators, and downstream targets. The balance between CDK activation and inactivation determines whether cells proceed through G1 into S phase, and from G2 to M, through regulatory mechanisms that are conserved in more complex eukaryotes. Many different stimuli exert checkpoint control including TGF, DNA damage, contact inhibition, replicative senescence, and growth factor withdrawal. G1 phase CDKs and their inhibitors (CKIs) are central to the pathways that regulate commitment to cellular division in response to positive as well as negative growth effectors. Many checkpoints are deregulated in oncogenesis, and this is often due to alterations in cyclin-CDK complexes.CDK activity is modulated by cyclin binding, phosphorylation, and CKIs, including the INK4 proteins and the closely related inhibitors p21Cip1 and p27Kip1. The downstream targets of CDKs and their modulation by TGF-beta and other growth factors include proteins of the retinoblastoma family, and the related modulation of the transcriptional activity of the E2F family members.
Cell cycle, or cell-division cycle, is the series of events that take place in a cell leading to its division and duplication (replication). Cell cycle control affects many aspects of development. Caenorhabditis elegans cell-cycle genes have been identified over the past decade, including at least two distinct Cyclin-Dependent Kinases (CDKs), their cyclin partners, positive and negative regulators, and downstream targets. The balance between CDK activation and inactivation determines whether cells proceed through G1 into S phase, and from G2 to M, through regulatory mechanisms that are conserved in more complex eukaryotes. Many different stimuli exert checkpoint control including TGF, DNA damage, contact inhibition, replicative senescence, and growth factor withdrawal. G1 phase CDKs and their inhibitors (CKIs) are central to the pathways that regulate commitment to cellular division in response to positive as well as negative growth effectors. Many checkpoints are deregulated in oncogenesis, and this is often due to alterations in cyclin-CDK complexes.CDK activity is modulated by cyclin binding, phosphorylation, and CKIs, including the INK4 proteins and the closely related inhibitors p21Cip1 and p27Kip1. The downstream targets of CDKs and their modulation by TGF-beta and other growth factors include proteins of the retinoblastoma family, and the related modulation of the transcriptional activity of the E2F family members.A disregulation of the cell cycle components may lead to tumor formation. As mentioned above, some genes like the cell cycle inhibitors, RB, p53 etc., when they mutate, may cause the cell to multiply uncontrollably, forming a tumor. The cells which are actively undergoing cell cycle are targeted in cancer therapy.
Selected Reviews:Dyson, N. (1998) The regulation of E2F by pRB-family proteins. Genes Dev. 12, 2245-2262.Elledge SJ. (1996) Cell cycle checkpoints: preventing an identity crisis. Science. 274(5293):1664-72.Ravitz MJ, Wenner CE. (1997)Cyclin-dependent kinase regulation during G1 phase and cell cycle regulation by TGF-beta. Adv Cancer Res. 71:165-207.van den Heuvel S.(2005) Cell-cycle regulation. WormBook. 21:1-16.癌症干细胞标志物
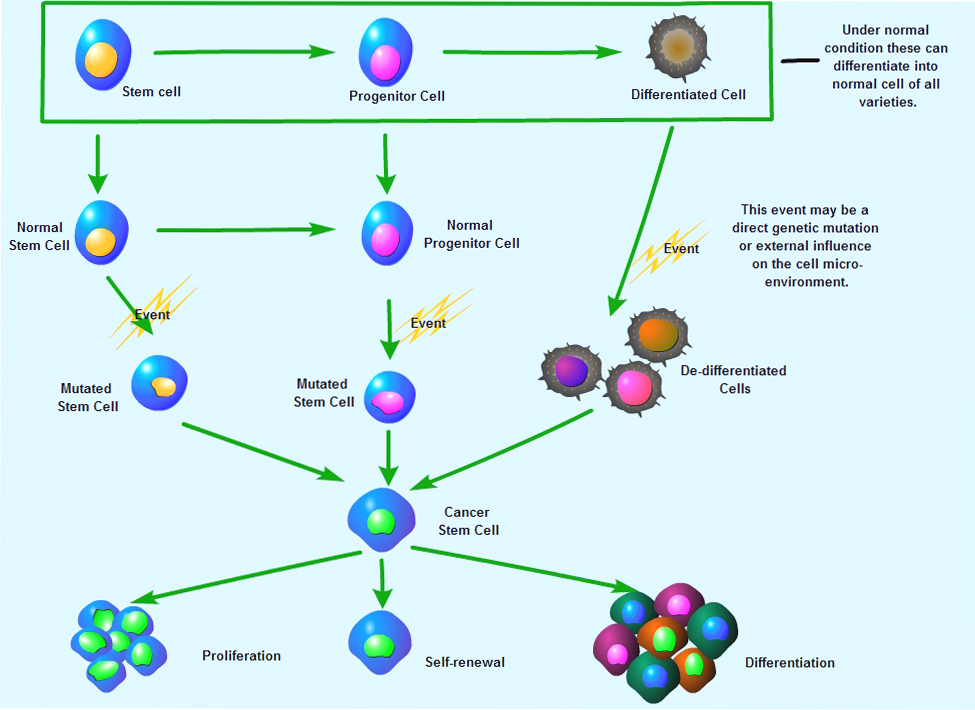 Cancer cells found within tumors or hematological cancers that possess characteristics associated with normal stem cells , specifically the ability to give rise to all cell types found in a particular cancer sample. CSCs are therefore tumorigenic (tumor-forming), perhaps in contrast to other non-tumorigenic cancer cells. CSCs may generate tumors through the stem cell processes of self-renewal and differentiation into multiple cell types. Such cells are proposed to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Therefore, development of specific therapies targeted at CSCs holds hope for improvement of survival and quality of life of cancer patients, especially for sufferers of metastatic disease .A number of studies have focused on identifying specific cancer stem cell markers. Pancreatic cancer stem cells express the surface markers CD44, CD24 and epithelial specific antigen (ESA). It has also been identified that liver progenitor cells share molecular markers with adult hepatocytes and fetal hepatocytes. In addition, markers frequently used to identify adult stem cells within the prostate, breast and intestine include CD44, CD133, ESA, CD69, p63, as well as some stem cell antigen, such as CD34, c-kit, Flt-3, NCAM, and Thy-1.Selected Reviews:Gupta PB (2009) Cancer stem cells: mirage or reality?. Nat Med 15 (9): 1010-2.Singh SK (2003) Identification of a cancer stem cell in human brain tumors. Cancer Research 63 (18): 5821-8Li C (2007) Identification of pancreatic cancer stem cells. Cancer Research 67 (3): 1030-7Dingli D. (2006) Successful therapy must eradicate cancer stem cells. Stem Cells 24, 2603-10.
Cancer cells found within tumors or hematological cancers that possess characteristics associated with normal stem cells , specifically the ability to give rise to all cell types found in a particular cancer sample. CSCs are therefore tumorigenic (tumor-forming), perhaps in contrast to other non-tumorigenic cancer cells. CSCs may generate tumors through the stem cell processes of self-renewal and differentiation into multiple cell types. Such cells are proposed to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Therefore, development of specific therapies targeted at CSCs holds hope for improvement of survival and quality of life of cancer patients, especially for sufferers of metastatic disease .A number of studies have focused on identifying specific cancer stem cell markers. Pancreatic cancer stem cells express the surface markers CD44, CD24 and epithelial specific antigen (ESA). It has also been identified that liver progenitor cells share molecular markers with adult hepatocytes and fetal hepatocytes. In addition, markers frequently used to identify adult stem cells within the prostate, breast and intestine include CD44, CD133, ESA, CD69, p63, as well as some stem cell antigen, such as CD34, c-kit, Flt-3, NCAM, and Thy-1.Selected Reviews:Gupta PB (2009) Cancer stem cells: mirage or reality?. Nat Med 15 (9): 1010-2.Singh SK (2003) Identification of a cancer stem cell in human brain tumors. Cancer Research 63 (18): 5821-8Li C (2007) Identification of pancreatic cancer stem cells. Cancer Research 67 (3): 1030-7Dingli D. (2006) Successful therapy must eradicate cancer stem cells. Stem Cells 24, 2603-10.癌症生物标志物
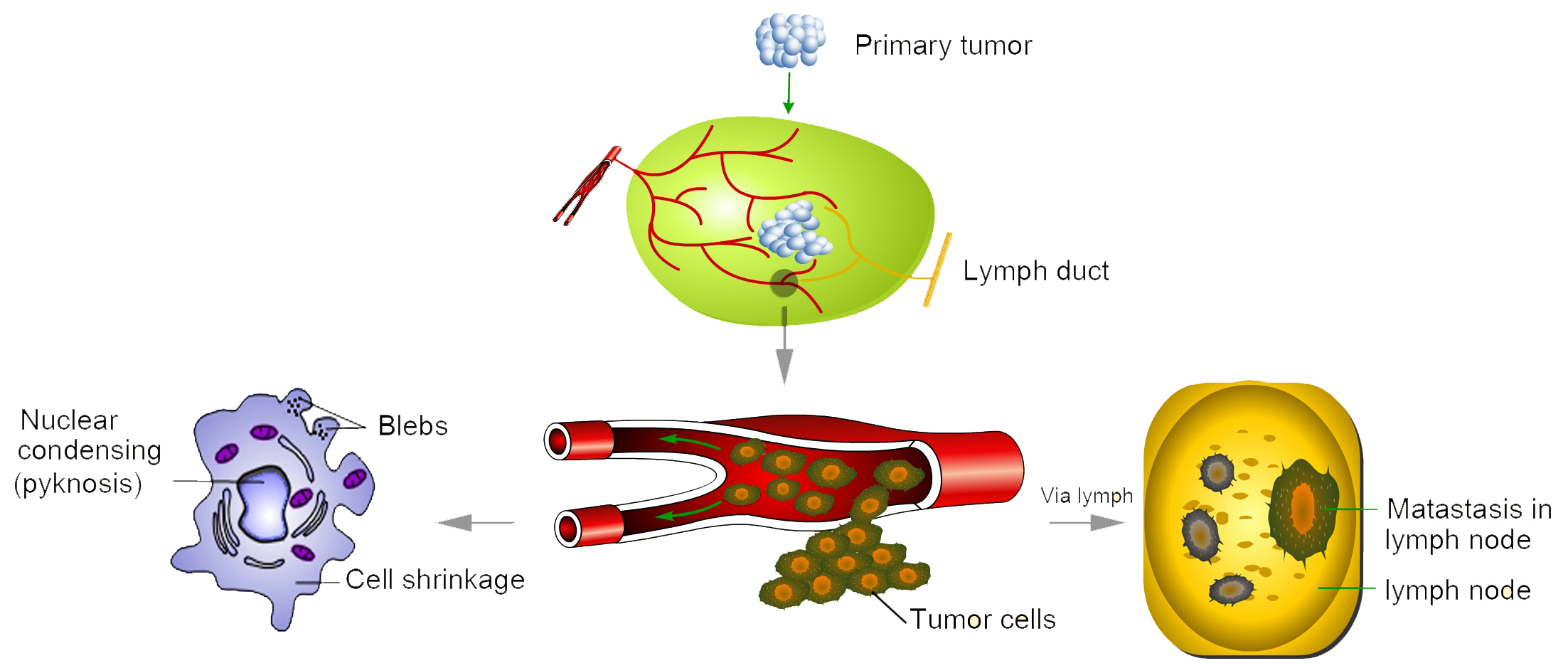 Cancer biomarkers are substances indicate that tumor state, progression characteristics, and response to therapies. Most cancer biomarkers are transcription factors, cell surface receptors, or secreted proteins that are produced by either cancer cells or other cells in response to cancer. They are present in tumor tissues or body fluids. Cancer biomarkers can be used for screening the general population, for differential diagnosis in symptomatic patients, and for clinical staging of cancer. Additionally, cancer biomarkers can be used to estimate tumor volume, to evaluate response to treatment, to assess disease recurrence through monitoring, or as prognostic indicators of disease progression. During the last decade, improved understanding of carcinogenesis and tumor progression has revealed a large number of potential cancer markers. It is predicted that even more will be discovered in the near future with the application of the new emerging genomics and proteomics technologies.
Cancer biomarkers are substances indicate that tumor state, progression characteristics, and response to therapies. Most cancer biomarkers are transcription factors, cell surface receptors, or secreted proteins that are produced by either cancer cells or other cells in response to cancer. They are present in tumor tissues or body fluids. Cancer biomarkers can be used for screening the general population, for differential diagnosis in symptomatic patients, and for clinical staging of cancer. Additionally, cancer biomarkers can be used to estimate tumor volume, to evaluate response to treatment, to assess disease recurrence through monitoring, or as prognostic indicators of disease progression. During the last decade, improved understanding of carcinogenesis and tumor progression has revealed a large number of potential cancer markers. It is predicted that even more will be discovered in the near future with the application of the new emerging genomics and proteomics technologies.
Selected Reviews:
Ludwig JA( 2005) Biomarkers in cancer staging, prognosis and treatment selection. Nat. Rev. Cancer 5 (11): 845-56.
Kulasingam V, et al. (2007) Tissue culture-based breast cancer biomarker discovery platform. Int J Cancer. 123(9):2007-12.
Singer E. A.(2007) PSA Screening and Elderly Men. JAMA 297 (9): 949
Jacobs Jon M.(2005) Utilizing Human Blood Plasma for Proteomic Biomarker Discovery. Journal of Proteome Research 4 (4): 1073-85细胞凋亡
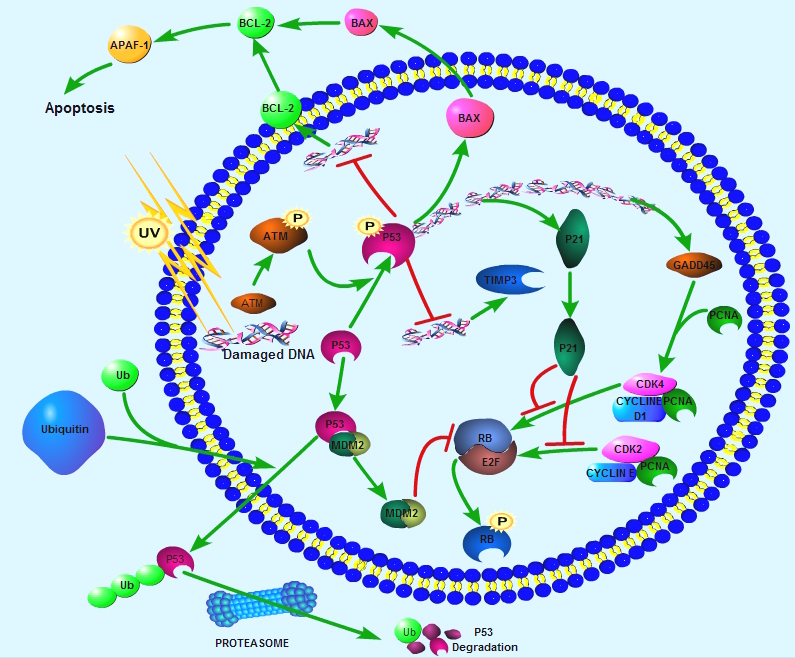
 Apoptosis is a naturally occurring process by which a cell is directed to Programmed Cell Death. Apoptosis is based on a genetic program that is an indispensable part of the development and function of an organism and is a regulated physiological process leading to cell death.In apoptosis pathways, signaling results in the activation of a family of Cysteine Proteases, named Caspases that act in a proteolytic cascade to dismantle and remove the dying cell, are central regulators of apoptosis. Initiator caspases (including 8, 9, 10) are closely coupled to pro-apototic signals. Once activated, these caspases cleave and activate downstream effector caspases (3, 6, 7) which in turn cleave cytoskeletal and nuclear proteins and induce apoptosis. Cytochrome C released from damaged mitochondria is coupled to the activation of caspase 9.Pro-apoptotic stimuli include the FasL, TNF, DNA damage. Fas and TNFR activate caspases 8 and 10; DNA damage leads to the activation of caspase 9. Anti-apoptotic ligands including growth factors and cytokines activate AKT and p90RSK, which inhibit Bad and prevent cytochrome C release. TNFR can also stimulate an anti-apoptotic pathway by inducing IAP, which directly inhibits caspases 3, 7 and 9.Alternatively, apoptosis is inhibited via an adaptor protein complex which activates NF-kB and induces survival genes including IAP. The Bcl-2 family of proteins regulate apoptosis by controlling mitochondrial permeability and the release of cytochrome C. The anti-apoptotic proteins Bcl-2 and Bcl-xL reside in the outer mitochondrial wall and inhibit cytochrome C release. The pro- apoptotic Bcl-2 proteins Bad, Bid, Bax and Bim reside in the cytosol but translocate to mitochondria following death signaling, where they promote the release of cytochrome C. Bad translocates to mitochondria and forms a pro-apoptotic complex with Bcl-xL. This translocation is inhibited by survival factors that induce the phosphorylation of Bad, leading to its cytosolic sequestration. Bax and Bim translocate to mitochondria in response to death stimuli, including survival factor withdrawal. Bcl-xL, Bcl-2 and Bax apparently influence the voltage-dependent anion channel (VDAC), which can control cytochrome C release. p53, activated following DNA damage, induces the transcription of Bax. Released cytochrome C binds Apaf1 and forms an activation complex with caspase9.Selected Reviews:Alberts Bruce (2008) Chapter 18 Apoptosis: Programmed Cell Death Eliminates Unwanted Cells. Molecular Biology of the Cell (textbook) (5th ed.). Garland Science. p. 1115. ISBN 978-0-8153-4105-5.Czerski L. (2004) Apoptosome formation and Caspase activation:is it different in the heart. J Mol Cardiol.37(13),643-652.Debatin K.M.(2004) Death receptors in chemotherapy and cancer. Oncogene 23(16), 2950-66.Lamkanfi M. (2007) Caspases in cell survival, proliferation and differentiation. Cell Death and Differentiation 14 (1): 44-55.
Apoptosis is a naturally occurring process by which a cell is directed to Programmed Cell Death. Apoptosis is based on a genetic program that is an indispensable part of the development and function of an organism and is a regulated physiological process leading to cell death.In apoptosis pathways, signaling results in the activation of a family of Cysteine Proteases, named Caspases that act in a proteolytic cascade to dismantle and remove the dying cell, are central regulators of apoptosis. Initiator caspases (including 8, 9, 10) are closely coupled to pro-apototic signals. Once activated, these caspases cleave and activate downstream effector caspases (3, 6, 7) which in turn cleave cytoskeletal and nuclear proteins and induce apoptosis. Cytochrome C released from damaged mitochondria is coupled to the activation of caspase 9.Pro-apoptotic stimuli include the FasL, TNF, DNA damage. Fas and TNFR activate caspases 8 and 10; DNA damage leads to the activation of caspase 9. Anti-apoptotic ligands including growth factors and cytokines activate AKT and p90RSK, which inhibit Bad and prevent cytochrome C release. TNFR can also stimulate an anti-apoptotic pathway by inducing IAP, which directly inhibits caspases 3, 7 and 9.Alternatively, apoptosis is inhibited via an adaptor protein complex which activates NF-kB and induces survival genes including IAP. The Bcl-2 family of proteins regulate apoptosis by controlling mitochondrial permeability and the release of cytochrome C. The anti-apoptotic proteins Bcl-2 and Bcl-xL reside in the outer mitochondrial wall and inhibit cytochrome C release. The pro- apoptotic Bcl-2 proteins Bad, Bid, Bax and Bim reside in the cytosol but translocate to mitochondria following death signaling, where they promote the release of cytochrome C. Bad translocates to mitochondria and forms a pro-apoptotic complex with Bcl-xL. This translocation is inhibited by survival factors that induce the phosphorylation of Bad, leading to its cytosolic sequestration. Bax and Bim translocate to mitochondria in response to death stimuli, including survival factor withdrawal. Bcl-xL, Bcl-2 and Bax apparently influence the voltage-dependent anion channel (VDAC), which can control cytochrome C release. p53, activated following DNA damage, induces the transcription of Bax. Released cytochrome C binds Apaf1 and forms an activation complex with caspase9.Selected Reviews:Alberts Bruce (2008) Chapter 18 Apoptosis: Programmed Cell Death Eliminates Unwanted Cells. Molecular Biology of the Cell (textbook) (5th ed.). Garland Science. p. 1115. ISBN 978-0-8153-4105-5.Czerski L. (2004) Apoptosome formation and Caspase activation:is it different in the heart. J Mol Cardiol.37(13),643-652.Debatin K.M.(2004) Death receptors in chemotherapy and cancer. Oncogene 23(16), 2950-66.Lamkanfi M. (2007) Caspases in cell survival, proliferation and differentiation. Cell Death and Differentiation 14 (1): 44-55.血管生成
.jpg) Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, and is a normal and vital process in growth and development, as well as in wound healing and in granulation tissue. Angiogenesis is regulated by multiple factors, including growth and differentiative factors, extracellular matrix components, membrane-bound receptors, intracellular signaling molecules, and angiogenesis inhibitors. Several growth factors, including FGFs (fibroblast growth factors), VEGFs (vascular endothelial growth factors), PDGFs, eprins, angiopoietins, TGF-beta and other participate in regulating angiogenesis. Angiogenesis inhibitors can be endogenous or exogenous. Endogenous angiogenesis inhibitors may be interleukins, interferons, chemokines, or growth factor regulators.In health, the body maintains a balance of angiogenesis regulators. However, the structures formed are often functionally abnormal, possibly due to an imbalance in the angiogenic process. Imbalance of angiogenesis stimulation and inhibition can lead to disease. In cancer, like tissues of the body, tumors need a blood supply. They get this through new blood vessel formation (angiogenesis). This is a fundamental step in the transition of tumors from a dormant state to a malignant one.Selected Reviews:Risau W. (1995) Vasculogenesis. Annual review of cell and developmental biology. 11: 73-91.Carmeliet P. (2005) Angiogenesis in life, disease and medicine. Nature. 438(7070):932-6.Tan A. (2010) Angiogenesis-inhibitors for metastatic thyroid cancer. Cochrane Database Syst Rev.3:CD007958.Flamme I. (1997) Molecular mechanisms of vasculogenesis and embryonic angiogenesis. Journal of cellular physiology 173 (2): 206-10.
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, and is a normal and vital process in growth and development, as well as in wound healing and in granulation tissue. Angiogenesis is regulated by multiple factors, including growth and differentiative factors, extracellular matrix components, membrane-bound receptors, intracellular signaling molecules, and angiogenesis inhibitors. Several growth factors, including FGFs (fibroblast growth factors), VEGFs (vascular endothelial growth factors), PDGFs, eprins, angiopoietins, TGF-beta and other participate in regulating angiogenesis. Angiogenesis inhibitors can be endogenous or exogenous. Endogenous angiogenesis inhibitors may be interleukins, interferons, chemokines, or growth factor regulators.In health, the body maintains a balance of angiogenesis regulators. However, the structures formed are often functionally abnormal, possibly due to an imbalance in the angiogenic process. Imbalance of angiogenesis stimulation and inhibition can lead to disease. In cancer, like tissues of the body, tumors need a blood supply. They get this through new blood vessel formation (angiogenesis). This is a fundamental step in the transition of tumors from a dormant state to a malignant one.Selected Reviews:Risau W. (1995) Vasculogenesis. Annual review of cell and developmental biology. 11: 73-91.Carmeliet P. (2005) Angiogenesis in life, disease and medicine. Nature. 438(7070):932-6.Tan A. (2010) Angiogenesis-inhibitors for metastatic thyroid cancer. Cochrane Database Syst Rev.3:CD007958.Flamme I. (1997) Molecular mechanisms of vasculogenesis and embryonic angiogenesis. Journal of cellular physiology 173 (2): 206-10.粘附分子
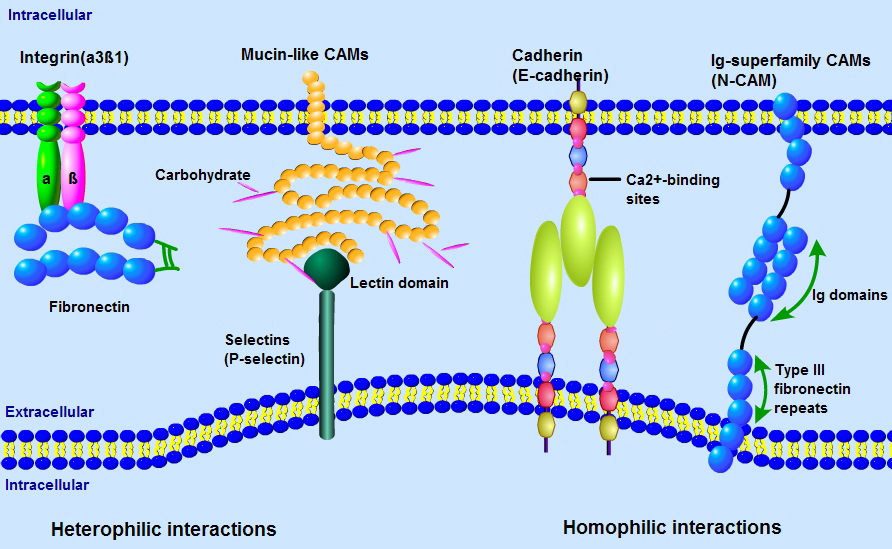 Adhesion Molecules are proteins located on the cell surface involved in binding with other cells or with the extracellular matrix (ECM) in the process of cell adhesion or cellular interactions. Essentially, adhesion molecules help cells stick to each other and to their surroundings. Adhesion Molecules include Integrins, Ig superfamily Cell Adhesion Molecules (CAMS), Cadherins, Lectins, and others. These proteins are typically transmembrane receptors and are composed of three domains: an intracellular domain that interacts with the cytoskeleton , a transmembrane domain, and an extracellular domain.Adhesion molecules play critical roles in a variety of biological processes. For instance, one important part adhesion molecules serve in the immune system is to enhance pairing between many less avid receptors and their ligands and transmit signals that direct specific effecter functions during an inflammatory response. In development they play key roles in tissue morphogenesis, cell migration, and axon guidance. Pathologically, adhesion molecules have been discovered to play a critical role in tumor cell invasiveness and metastases.Selected Reviews:Angst B. (2001)The cadherin superfamily: diversity in form and function. J Cell Sci. 114 (Pt 4): 629-41Zetter BR. (1993) Adhesion molecules in tumor metastasis. Semin Cancer Biol. 4(4):219-29.Hulpiau P. (2009)Molecular evolution of the cadherin superfamily. Int. J. Biochem. Cell Biol.. 41 (2): 349-69.Ikeda H. (1998) Cell adhesion molecule. Nippon Rinsho. 56(10):2493-9.
Adhesion Molecules are proteins located on the cell surface involved in binding with other cells or with the extracellular matrix (ECM) in the process of cell adhesion or cellular interactions. Essentially, adhesion molecules help cells stick to each other and to their surroundings. Adhesion Molecules include Integrins, Ig superfamily Cell Adhesion Molecules (CAMS), Cadherins, Lectins, and others. These proteins are typically transmembrane receptors and are composed of three domains: an intracellular domain that interacts with the cytoskeleton , a transmembrane domain, and an extracellular domain.Adhesion molecules play critical roles in a variety of biological processes. For instance, one important part adhesion molecules serve in the immune system is to enhance pairing between many less avid receptors and their ligands and transmit signals that direct specific effecter functions during an inflammatory response. In development they play key roles in tissue morphogenesis, cell migration, and axon guidance. Pathologically, adhesion molecules have been discovered to play a critical role in tumor cell invasiveness and metastases.Selected Reviews:Angst B. (2001)The cadherin superfamily: diversity in form and function. J Cell Sci. 114 (Pt 4): 629-41Zetter BR. (1993) Adhesion molecules in tumor metastasis. Semin Cancer Biol. 4(4):219-29.Hulpiau P. (2009)Molecular evolution of the cadherin superfamily. Int. J. Biochem. Cell Biol.. 41 (2): 349-69.Ikeda H. (1998) Cell adhesion molecule. Nippon Rinsho. 56(10):2493-9.如何挑选抗体
No, we are not a shop. We are a price comparison shopping and travel website. We help you to get the best deals, by comparing the prices of retailers and travel suppliers for you. We research millions of products and thousands of suppliers so you don't have to.
Once you decide you would like to buy a product, you click through directly to the supplier's website. You will deal directly with the retailer or travel supplier after that stage. If you have any problems with an order you should contact the supplier



